当前位置:
X-MOL 学术
›
Mol. Microbiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Characterization of a small tRNA-binding protein that interacts with the archaeal proteasome complex
Molecular Microbiology ( IF 2.6 ) Pub Date : 2022-05-26 , DOI: 10.1111/mmi.14948 Gaëlle Hogrel 1 , Laura Marino-Puertas 1 , Sébastien Laurent 2 , Ziad Ibrahim 1 , Jacques Covès 1 , Eric Girard 1 , Frank Gabel 1 , Daphna Fenel 1 , Marie-Claire Daugeron 3 , Béatrice Clouet-d'Orval 4 , Tamara Basta 3 , Didier Flament 2 , Bruno Franzetti 1
Molecular Microbiology ( IF 2.6 ) Pub Date : 2022-05-26 , DOI: 10.1111/mmi.14948 Gaëlle Hogrel 1 , Laura Marino-Puertas 1 , Sébastien Laurent 2 , Ziad Ibrahim 1 , Jacques Covès 1 , Eric Girard 1 , Frank Gabel 1 , Daphna Fenel 1 , Marie-Claire Daugeron 3 , Béatrice Clouet-d'Orval 4 , Tamara Basta 3 , Didier Flament 2 , Bruno Franzetti 1
Affiliation
|
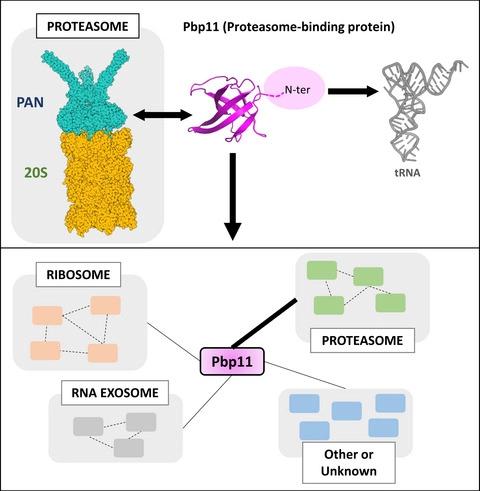
The proteasome system allows the elimination of functional or structurally impaired proteins. This includes the degradation of nascent peptides. In Archaea, how the proteasome complex interacts with the translational machinery remains to be described. Here, we characterized a small orphan protein, Q9UZY3 (UniProt ID), conserved in Thermococcales. The protein was identified in native pull-down experiments using the proteasome regulatory complex (proteasome-activating nucleotidase [PAN]) as bait. X-ray crystallography and small-angle X-ray scattering experiments revealed that the protein is monomeric and adopts a β-barrel core structure with an oligonucleotide/oligosaccharide-binding (OB)-fold, typically found in translation elongation factors. Mobility shift experiment showed that Q9UZY3 displays transfer ribonucleic acid (tRNA)-binding properties. Pull-downs, co-immunoprecipitation and isothermal titration calorimetry (ITC) studies revealed that Q9UZY3 interacts in vitro with PAN. Native pull-downs and proteomic analysis using different versions of Q9UZY3 showed that the protein interacts with the assembled PAN–20S proteasome machinery in Pyrococcus abyssi (Pa) cellular extracts. The protein was therefore named Pbp11, for Proteasome-Binding Protein of 11 kDa. Interestingly, the interaction network of Pbp11 also includes ribosomal proteins, tRNA-processing enzymes and exosome subunits dependent on Pbp11's N-terminal domain that was found to be essential for tRNA binding. Together these data suggest that Pbp11 participates in an interface between the proteasome and the translational machinery.
中文翻译:

与古细菌蛋白酶体复合物相互作用的小 tRNA 结合蛋白的表征
蛋白酶体系统允许消除功能性或结构受损的蛋白质。这包括新生肽的降解。在古生菌中,蛋白酶体复合物如何与翻译机制相互作用仍有待描述。在这里,我们描述了一种在 Thermococcales 中保守的小型孤儿蛋白 Q9UZY3 (UniProt ID)。该蛋白质是在使用蛋白酶体调节复合物(蛋白酶体激活核苷酸酶 [PAN])作为诱饵的天然下拉实验中鉴定的。X 射线晶体学和小角 X 射线散射实验表明,该蛋白质是单体并采用具有寡核苷酸/寡糖结合 (OB) 折叠的 β 桶核心结构,通常存在于翻译延伸因子中。迁移率变化实验表明 Q9UZY3 具有转移核糖核酸 (tRNA) 结合特性。下拉、免疫共沉淀和等温滴定量热 (ITC) 研究表明 Q9UZY3 在体外与 PAN 相互作用。使用不同版本的 Q9UZY3 的天然下拉和蛋白质组学分析表明,该蛋白质与组装的 PAN-20S 蛋白酶体机制相互作用Pyrococcus abyssi (Pa) 细胞提取物。因此,该蛋白质被命名为 Pbp11,即 11 kDa 的蛋白酶体结合蛋白。有趣的是,Pbp11 的相互作用网络还包括依赖于 Pbp11 的 N 端结构域的核糖体蛋白、tRNA 加工酶和外泌体亚基,这些结构域被发现对 tRNA 结合至关重要。这些数据一起表明 Pbp11 参与了蛋白酶体和翻译机制之间的接口。
更新日期:2022-05-26
中文翻译:

与古细菌蛋白酶体复合物相互作用的小 tRNA 结合蛋白的表征
蛋白酶体系统允许消除功能性或结构受损的蛋白质。这包括新生肽的降解。在古生菌中,蛋白酶体复合物如何与翻译机制相互作用仍有待描述。在这里,我们描述了一种在 Thermococcales 中保守的小型孤儿蛋白 Q9UZY3 (UniProt ID)。该蛋白质是在使用蛋白酶体调节复合物(蛋白酶体激活核苷酸酶 [PAN])作为诱饵的天然下拉实验中鉴定的。X 射线晶体学和小角 X 射线散射实验表明,该蛋白质是单体并采用具有寡核苷酸/寡糖结合 (OB) 折叠的 β 桶核心结构,通常存在于翻译延伸因子中。迁移率变化实验表明 Q9UZY3 具有转移核糖核酸 (tRNA) 结合特性。下拉、免疫共沉淀和等温滴定量热 (ITC) 研究表明 Q9UZY3 在体外与 PAN 相互作用。使用不同版本的 Q9UZY3 的天然下拉和蛋白质组学分析表明,该蛋白质与组装的 PAN-20S 蛋白酶体机制相互作用Pyrococcus abyssi (Pa) 细胞提取物。因此,该蛋白质被命名为 Pbp11,即 11 kDa 的蛋白酶体结合蛋白。有趣的是,Pbp11 的相互作用网络还包括依赖于 Pbp11 的 N 端结构域的核糖体蛋白、tRNA 加工酶和外泌体亚基,这些结构域被发现对 tRNA 结合至关重要。这些数据一起表明 Pbp11 参与了蛋白酶体和翻译机制之间的接口。











































 京公网安备 11010802027423号
京公网安备 11010802027423号