Molecular Therapy ( IF 12.1 ) Pub Date : 2022-05-26 , DOI: 10.1016/j.ymthe.2022.05.021 Jinna Wu 1 , Yuyu Chen 1 , Zhiheng Liao 1 , Hengyu Liu 1 , Shun Zhang 1 , Dongmei Zhong 2 , Xianjian Qiu 3 , Taiqiu Chen 3 , Deying Su 4 , Xiaona Ke 1 , Yong Wan 1 , Taifeng Zhou 1 , Peiqiang Su 1
|
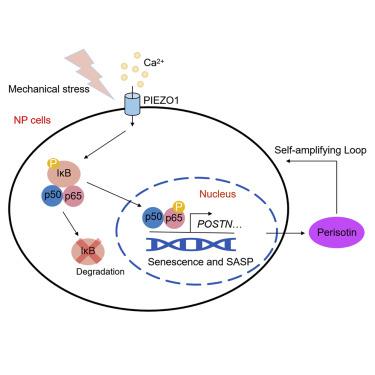
Abnormal mechanical load is a main risk factor of intervertebral disc degeneration (IDD), and cellular senescence is a pathological change in IDD. In addition, extracellular matrix (ECM) stiffness promotes human nucleus pulposus cells (hNPCs) senescence. However, the molecular mechanism underlying mechano-induced cellular senescence and IDD progression is not yet fully elucidated. First, we demonstrated that mechano-stress promoted hNPCs senescence via NF-κB signaling. Subsequently, we identified periostin as the main mechano-responsive molecule in hNPCs through unbiased sequencing, which was transcriptionally upregulated by NF-κB p65; moreover, secreted periostin by senescent hNPCs further promoted senescence and upregulated the catabolic process in hNPCs through activating NF-κB, forming a positive loop. Both Postn (encoding periostin) knockdown via siRNA and periostin inactivation via neutralizing antibodies alleviated IDD and NPCs senescence. Furthermore, we found that mechano-stress initiated the positive feedback of NF-κB and periostin via PIEZO1. PIEZO1 activation by Yoda1 induced severe IDD in rat tails without compression, and Postn knockdown alleviated the Yoda1-induced IDD in vivo. Here, we reported for the first time that self-amplifying loop of NF-κB and periostin initiated via PIEZO1 under mechano-stress accelerated NPCs senescence, leading to IDD. Furthermore, periostin neutralizing antibodies, which may serve as potential therapeutic agents for IDD, interrupted this loop.
中文翻译:

PIEZO1启动的NF-κB和骨膜素的自放大环加速机械诱导的髓核细胞衰老和椎间盘退变
机械负荷异常是椎间盘退变(IDD)的主要危险因素,细胞衰老是IDD的病理改变。此外,细胞外基质(ECM)硬度会促进人髓核细胞(hNPC)衰老。然而,机械诱导细胞衰老和 IDD 进展的分子机制尚未完全阐明。首先,我们证明机械应激通过 NF-κB 信号传导促进 hNPC 衰老。随后,我们通过无偏测序确定了骨膜素是 hNPC 中主要的机械响应分子,其转录受到 NF-κB p65 的上调;此外,衰老hNPCs分泌的骨膜素通过激活NF-κB进一步促进衰老并上调hNPCs的分解代谢过程,形成正循环。通过 siRNA 敲低Postn (编码骨膜素)和通过中和抗体灭活骨膜素均可减轻 IDD 和 NPC 的衰老。此外,我们发现机械应力通过 PIEZO1 启动 NF-κB 和骨膜素的正反馈。Yoda1 激活 PIEZO1 在未受压的情况下诱导大鼠尾部严重 IDD,而Postn敲低则减轻了 Yoda1 诱导的体内IDD 。在这里,我们首次报道了在机械应力下通过 PIEZO1 启动的 NF-κB 和骨膜素的自我放大环加速了 NPC 衰老,导致 IDD。此外,骨膜素中和抗体(可能作为 IDD 的潜在治疗剂)中断了这一循环。











































 京公网安备 11010802027423号
京公网安备 11010802027423号