Cell Host & Microbe ( IF 20.6 ) Pub Date : 2022-05-26 , DOI: 10.1016/j.chom.2022.05.002 Henry H Le 1 , Min-Ting Lee 1 , Kevin R Besler 1 , Elizabeth L Johnson 1
|
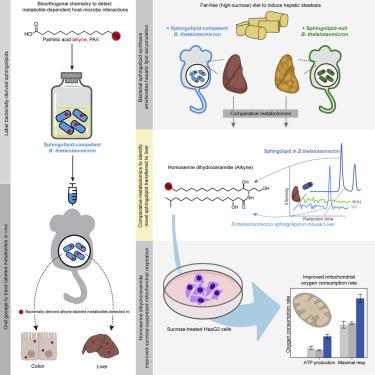
Microbially-derived gut metabolites are important contributors to host phenotypes, many of which may link microbiome composition to metabolic disease. However, relatively few metabolites with known bioactivity have been traced from specific microbes to host tissues. Here, we use a labeling strategy to characterize and trace bacterial sphingolipids from the gut symbiont Bacteroides thetaiotaomicron to mouse colons and livers. We find that bacterial sphingolipid synthesis rescues excess lipid accumulation in a mouse model of hepatic steatosis and observe the transit of a previously uncharacterized bacterial sphingolipid to the liver. The addition of this sphingolipid to hepatocytes improves respiration in response to fatty-acid overload, suggesting that sphingolipid transfer to the liver could potentially contribute to microbiota-mediated liver function. This work establishes a role for bacterial sphingolipids in modulating hepatic phenotypes and defines a workflow that permits the characterization of other microbial metabolites with undefined functions in host health.
中文翻译:

宿主肝脏代谢受肠道微生物群衍生的鞘脂调节
微生物来源的肠道代谢物是宿主表型的重要贡献者,其中许多可能将微生物组组成与代谢疾病联系起来。然而,从特定微生物追踪到宿主组织的具有已知生物活性的代谢物相对较少。在这里,我们使用标记策略来表征和追踪肠道共生体Bacteroides thetaiotaomicron中的细菌鞘脂小鼠结肠和肝脏。我们发现细菌鞘脂合成可以挽救肝脏脂肪变性小鼠模型中的过量脂质积累,并观察到先前未表征的细菌鞘脂向肝脏的转运。将这种鞘脂添加到肝细胞中可以改善对脂肪酸超载的呼吸反应,这表明鞘脂转移到肝脏可能有助于微生物群介导的肝功能。这项工作确定了细菌鞘脂在调节肝脏表型中的作用,并定义了一个工作流程,该工作流程允许表征在宿主健康中具有未定义功能的其他微生物代谢物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号