当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ylide-Functionalized Diisopropyl Phosphine (prYPhos): A Ligand for Selective Suzuki-Miyaura Couplings of Aryl Chlorides
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2022-05-25 , DOI: 10.1002/adsc.202200321 Xiao-Jing Wei 1 , Bingxiang Xue 1 , Jens Handelmann 1 , Zhiyong Hu 1 , Heidar Darmandeh 1 , Viktoria Däschlein-Gessner 2 , Lukas J. Goossen 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2022-05-25 , DOI: 10.1002/adsc.202200321 Xiao-Jing Wei 1 , Bingxiang Xue 1 , Jens Handelmann 1 , Zhiyong Hu 1 , Heidar Darmandeh 1 , Viktoria Däschlein-Gessner 2 , Lukas J. Goossen 1
Affiliation
|
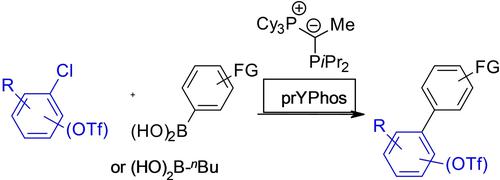
The ylide-functionalized diisopropyl phosphine (prYPhos) promotes Pd-catalyzed Suzuki-Miyaura couplings of aryl chlorides and bromides with arylboronic acids. Using Pd2(dba)3/prYPhos as the catalyst and K2CO3 as the base, various (hetero)biaryls were synthesized in 45–98% yields at mild reaction conditions, tolerating sensitive functional groups and steric hindrance. The electron rich, sterically demanding ligand induces a high selectivity for halides versus triflate. Chloroaryl triflates were selectively coupled at the chloride, leaving inherently more reactive triflate groups in place.
中文翻译:

叶立德官能化二异丙基膦 (prYPhos):芳基氯化物选择性 Suzuki-Miyaura 偶联的配体
叶立德官能化的二异丙基膦 (prYPhos) 可促进 Pd 催化的芳基氯化物和溴化物与芳基硼酸的 Suzuki-Miyaura 偶联。使用 Pd 2 (dba) 3 /prYPhos 作为催化剂和 K 2 CO 3作为碱,在温和的反应条件下以 45-98% 的收率合成了各种(杂)联芳基化合物,耐受敏感的官能团和空间位阻。富电子、空间要求高的配体对卤化物和三氟甲磺酸盐具有较高的选择性。氯芳基三氟甲磺酸酯在氯化物处选择性偶联,留下固有的更具反应性的三氟甲磺酸酯基团。
更新日期:2022-05-25
中文翻译:

叶立德官能化二异丙基膦 (prYPhos):芳基氯化物选择性 Suzuki-Miyaura 偶联的配体
叶立德官能化的二异丙基膦 (prYPhos) 可促进 Pd 催化的芳基氯化物和溴化物与芳基硼酸的 Suzuki-Miyaura 偶联。使用 Pd 2 (dba) 3 /prYPhos 作为催化剂和 K 2 CO 3作为碱,在温和的反应条件下以 45-98% 的收率合成了各种(杂)联芳基化合物,耐受敏感的官能团和空间位阻。富电子、空间要求高的配体对卤化物和三氟甲磺酸盐具有较高的选择性。氯芳基三氟甲磺酸酯在氯化物处选择性偶联,留下固有的更具反应性的三氟甲磺酸酯基团。











































 京公网安备 11010802027423号
京公网安备 11010802027423号