当前位置:
X-MOL 学术
›
Propellants Explos. Pyrotech.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Theoretical Research on the Combustion Mechanism and Kinetics for Aluminum and Carbon Dioxide
Propellants, Explosives, Pyrotechnics ( IF 1.7 ) Pub Date : 2022-05-26 , DOI: 10.1002/prep.202100248 Siyi Zhang 1 , Yunlan Sun 1 , Baozhong Zhu 1 , Tao Sun 2
Propellants, Explosives, Pyrotechnics ( IF 1.7 ) Pub Date : 2022-05-26 , DOI: 10.1002/prep.202100248 Siyi Zhang 1 , Yunlan Sun 1 , Baozhong Zhu 1 , Tao Sun 2
Affiliation
|
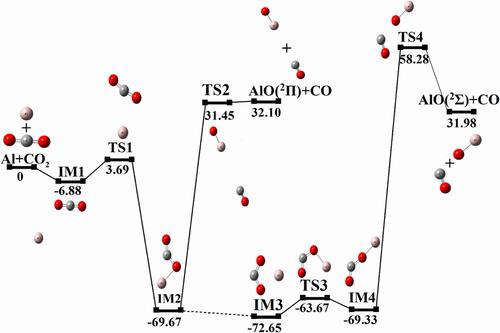
The reaction mechanism and kinetics of ground state aluminum (Al) atom and carbon dioxide (CO2) molecule were studied by quantum chemical calculations. The transition states and reaction paths were investigated at the B3LYP/6-311+G(d,p) (Becke type three-parameter Lee-Yang-Parr functional model) basis set. The energy of each stagnation point was calculated at the high-precision CBS-QB3 (limiting complete basis set) level of theory. The reaction of CO2 with Al is a complex process. There are four basic reaction processes and two main reaction paths (Reaction pathway 1: Al+CO2→IM1→TS1→IM2→TS2→AlO (2Π)+CO and Reaction pathway 2: Al+CO2→IM3→TS3→AlCO2linear→TS4→AlO (2Σ)+CO). The temperature- and pressure-dependent rate constants of the reaction of Al and CO2 was estimated by CBS-QB3//B3LYP/6-311+G(d,p) level at 300∼3000 K, which is combined with Transition State Theory (TST), Variational Transition State Theory (VTST), and Rice-Ramsperger-Kassel-Marcus (RRKM) theory. The total rate constant of Reaction pathway 1 (ktotal,1) is much higher than that of Reaction pathway 2 (ktotal,2), indicating that the reaction of Al with CO2 is easier to occur through Reaction pathway 1. Compared with ktotal,2, ktotal,1 shows a strong pressure dependence at high temperature, but it is almost independent with the pressure at low temperature.
中文翻译:

铝和二氧化碳燃烧机理与动力学的理论研究
通过量子化学计算研究了基态铝(Al)原子与二氧化碳(CO 2 )分子的反应机理和动力学。在 B3LYP/6-311+G(d,p)(Becke 型三参数 Lee-Yang-Parr 函数模型)基组中研究了过渡态和反应路径。每个驻点的能量是在高精度CBS-QB3(极限完全基组)理论水平上计算的。CO 2与Al的反应是一个复杂的过程。有四种基本反应过程和两条主要反应路径(反应路径1:Al+CO 2 →IM1→TS1→IM2→TS2→AlO ( 2 Π)+CO 和反应路径2:Al+CO 2 →IM3→TS3→ AlCO 2线性→TS4→AlO (2Σ) + CO)。通过 CBS-QB3//B3LYP/6-311+G(d,p) 水平在 300∼3000 K 下估计 Al 和 CO 2反应的温度和压力依赖性速率常数,并结合过渡态理论 (TST)、变分过渡态理论 (VTST) 和 Rice-Ramsperger-Kassel-Marcus (RRKM) 理论。反应途径 1 的总速率常数 ( k total,1 ) 远高于反应途径 2 ( k total,2 ),说明 Al 与 CO 2的反应更容易通过反应途径 1 发生。k总计,2 , k总计,1在高温下表现出强烈的压力依赖性,但在低温下几乎与压力无关。
更新日期:2022-05-26
中文翻译:

铝和二氧化碳燃烧机理与动力学的理论研究
通过量子化学计算研究了基态铝(Al)原子与二氧化碳(CO 2 )分子的反应机理和动力学。在 B3LYP/6-311+G(d,p)(Becke 型三参数 Lee-Yang-Parr 函数模型)基组中研究了过渡态和反应路径。每个驻点的能量是在高精度CBS-QB3(极限完全基组)理论水平上计算的。CO 2与Al的反应是一个复杂的过程。有四种基本反应过程和两条主要反应路径(反应路径1:Al+CO 2 →IM1→TS1→IM2→TS2→AlO ( 2 Π)+CO 和反应路径2:Al+CO 2 →IM3→TS3→ AlCO 2线性→TS4→AlO (2Σ) + CO)。通过 CBS-QB3//B3LYP/6-311+G(d,p) 水平在 300∼3000 K 下估计 Al 和 CO 2反应的温度和压力依赖性速率常数,并结合过渡态理论 (TST)、变分过渡态理论 (VTST) 和 Rice-Ramsperger-Kassel-Marcus (RRKM) 理论。反应途径 1 的总速率常数 ( k total,1 ) 远高于反应途径 2 ( k total,2 ),说明 Al 与 CO 2的反应更容易通过反应途径 1 发生。k总计,2 , k总计,1在高温下表现出强烈的压力依赖性,但在低温下几乎与压力无关。











































 京公网安备 11010802027423号
京公网安备 11010802027423号