当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Markov State Modeling Analysis Captures Changes in the Temperature-Sensitive N-Terminal and β-Turn Regions of the p53 DNA-Binding Domain
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2022-05-25 , DOI: 10.1021/acs.jcim.2c00380 Shruti Koulgi 1 , Archana Achalere 1 , Uddhavesh Sonavane 1 , Rajendra Joshi 1
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2022-05-25 , DOI: 10.1021/acs.jcim.2c00380 Shruti Koulgi 1 , Archana Achalere 1 , Uddhavesh Sonavane 1 , Rajendra Joshi 1
Affiliation
|
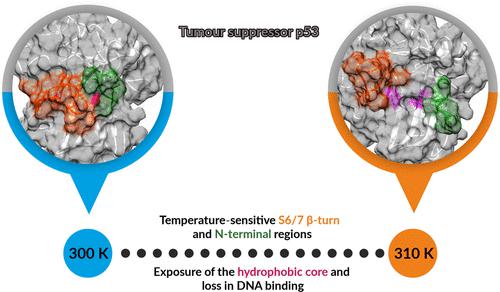
The transcription factor p53 is one of the most widely studied cancer proteins. Its temperature-sensitive nature suggests reduction in functionality at physiological temperatures. Temperature-induced conformational variations and their impact on its functional ability still remain unexplored. A total of 20.8 μs molecular dynamics simulations of wildtype p53 in the apo and the DNA-bound states have been performed at 300 K and 310 K. Further, Markov State Modeling (MSM) analyses were performed, considering Cα–Cα distances as reaction coordinates. Filtering of these distances based on correlation with the time-independent components (tICs) resulted in 16 and 32 distances for apo and DNA-bound systems, respectively. Individual MSM analyses using these filtered distances were performed for both p53 systems. These Cα–Cα residue pairs belonged to the N-terminal, S6/7 β-turn, loop L2, loop L3, and hydrophobic core residues. At physiological temperatures, apo-p53 exhibits exposure of its hydrophobic core, where the temperature-sensitive hotspot residues were also located. This exposure was the result of the S6/7 β-turn and N-terminal moving apart. In the DNA-bound p53 system, loop L1 attains an open conformation at physiological temperatures, which weakens the DNA binding. It is already known that p53 mutants that lack DNA binding also tend to show similar conformational variations. The S6/7 β-turn along with the already known functionally important loop L2 may pose as regions to be targeted to overcome the loss in binding of temperature-sensitive wildtype p53. Rescue strategies directed toward these temperature-sensitive regions may be useful to recuperate its strong binding at physiological temperatures.
中文翻译:

马尔可夫状态建模分析捕获 p53 DNA 结合域的温度敏感 N 末端和 β 转角区域的变化
转录因子 p53 是研究最广泛的癌症蛋白之一。其对温度敏感的性质表明在生理温度下功能会降低。温度诱导的构象变化及其对其功能的影响仍未得到探索。在 300 K 和 310 K 下对 apo 中的野生型 p53 和 DNA 结合状态进行了总共 20.8 μs 的分子动力学模拟。此外,考虑到 C α –C α ,进行了马尔可夫状态建模 (MSM) 分析距离作为反应坐标。根据与时间无关的成分 (tIC) 的相关性对这些距离进行过滤,分别为 apo 和 DNA 结合系统产生 16 和 32 个距离。对两个 p53 系统进行了使用这些过滤距离的单独 MSM 分析。这些 C α –C α残基对属于 N 末端、S6/7 β-转角、环 L2、环 L3 和疏水核心残基。在生理温度下,apo-p53 会暴露其疏水核心,温度敏感的热点残基也位于此处。这种暴露是 S6/7 β 转角和 N 末端分开的结果。在 DNA 结合的 p53 系统中,环 L1 在生理温度下达到开放构象,这削弱了 DNA 结合。众所周知,缺乏 DNA 结合的 p53 突变体也倾向于表现出相似的构象变异。S6/7 β 转角以及已知的功能重要的环 L2 可能构成目标区域,以克服温度敏感的野生型 p53 的结合损失。
更新日期:2022-05-25
中文翻译:

马尔可夫状态建模分析捕获 p53 DNA 结合域的温度敏感 N 末端和 β 转角区域的变化
转录因子 p53 是研究最广泛的癌症蛋白之一。其对温度敏感的性质表明在生理温度下功能会降低。温度诱导的构象变化及其对其功能的影响仍未得到探索。在 300 K 和 310 K 下对 apo 中的野生型 p53 和 DNA 结合状态进行了总共 20.8 μs 的分子动力学模拟。此外,考虑到 C α –C α ,进行了马尔可夫状态建模 (MSM) 分析距离作为反应坐标。根据与时间无关的成分 (tIC) 的相关性对这些距离进行过滤,分别为 apo 和 DNA 结合系统产生 16 和 32 个距离。对两个 p53 系统进行了使用这些过滤距离的单独 MSM 分析。这些 C α –C α残基对属于 N 末端、S6/7 β-转角、环 L2、环 L3 和疏水核心残基。在生理温度下,apo-p53 会暴露其疏水核心,温度敏感的热点残基也位于此处。这种暴露是 S6/7 β 转角和 N 末端分开的结果。在 DNA 结合的 p53 系统中,环 L1 在生理温度下达到开放构象,这削弱了 DNA 结合。众所周知,缺乏 DNA 结合的 p53 突变体也倾向于表现出相似的构象变异。S6/7 β 转角以及已知的功能重要的环 L2 可能构成目标区域,以克服温度敏感的野生型 p53 的结合损失。











































 京公网安备 11010802027423号
京公网安备 11010802027423号