当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
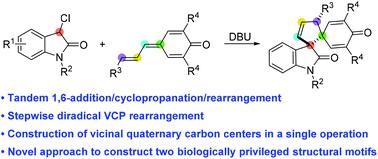
Tandem 1,6-addition/cyclopropanation/rearrangement reaction of vinylogous para-quinone methides with 3-chlorooxindoles: construction of vicinal quaternary carbon centers
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2022-05-25 , DOI: 10.1039/d2qo00471b Yuan Pan 1 , Weiwu Ren 1, 2 , Zhanhao Zhang 3 , Fengbiao Luo 1 , Xiaohan Hou 1 , Xiaoyang Li 1 , Yun-Fang Yang 3 , Yang Wang 1, 2
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2022-05-25 , DOI: 10.1039/d2qo00471b Yuan Pan 1 , Weiwu Ren 1, 2 , Zhanhao Zhang 3 , Fengbiao Luo 1 , Xiaohan Hou 1 , Xiaoyang Li 1 , Yun-Fang Yang 3 , Yang Wang 1, 2
Affiliation
|
A novel tandem 1,6-addition/cyclopropanation/rearrangement reaction of vinylogous para-quinone methides with 3-chlorooxindoles is reported. This method provides an efficient approach to various functionalized dispirooxindole–cyclopentane–cyclohexadienones with vicinal quaternary carbon centers. Computational studies have been conducted to provide insight into the origin of diastereoselectivity and predict a stepwise diradical mechanism. The anti-tumor activities of these structurally unique dispirooxindole derivatives are also reported.
中文翻译:

插烯对醌甲基化物与3-氯羟吲哚的串联1,6-加成/环丙烷化/重排反应:邻位季碳中心的构建
报道了一种新的串联 1,6-加成/环丙烷化/重排反应的乙烯基对醌甲基化物与 3-氯羟吲哚。该方法为具有邻位季碳中心的各种官能化双螺环吲哚-环戊烷-环己二烯酮提供了一种有效的方法。已经进行了计算研究以深入了解非对映选择性的起源并预测逐步的双自由基机制。还报道了这些结构独特的双螺吲哚衍生物的抗肿瘤活性。
更新日期:2022-05-25
中文翻译:

插烯对醌甲基化物与3-氯羟吲哚的串联1,6-加成/环丙烷化/重排反应:邻位季碳中心的构建
报道了一种新的串联 1,6-加成/环丙烷化/重排反应的乙烯基对醌甲基化物与 3-氯羟吲哚。该方法为具有邻位季碳中心的各种官能化双螺环吲哚-环戊烷-环己二烯酮提供了一种有效的方法。已经进行了计算研究以深入了解非对映选择性的起源并预测逐步的双自由基机制。还报道了这些结构独特的双螺吲哚衍生物的抗肿瘤活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号