Chem ( IF 19.1 ) Pub Date : 2022-05-23 , DOI: 10.1016/j.chempr.2022.04.025 Wenju Chang , Yu Chen , Shuo Lu , Hongyun Jiao , Yajun Wang , Tianyu Zheng , Zhuangzhi Shi , Yingbin Han , Yi Lu , Yi Wang , Yi Pan , Jin-Quan Yu , Kendall N. Houk , Fang Liu , Yong Liang
|
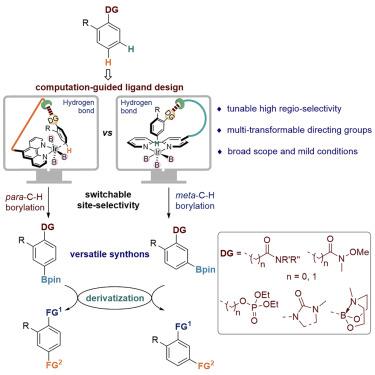
An ideal way to synthesize multi-substituted arenes is the selective installation of any group on any position of aromatic rings with any substituent. However, reactant-controlled selective functionalization of both meta- and para-C–H bonds is essentially impossible because of their intrinsically contradictory electronic and steric demands. Here, we report the first examples of catalysts that can direct the tunable borylation of a variety of arenes with precise control at either meta or para site through the computational design of ligands. A wide range of hydrogen bond acceptors can serve as directing groups, including Weinreb amides, phosphonates, and boronates, which can be easily transformed into many different types of functional groups. This work also showcases the critical role of transition-state calculations in catalyst design for remote C–H activation.
中文翻译:

计算设计的配体使芳烃中远程 C-H 键的可调谐硼化成为可能
合成多取代芳烃的理想方法是在具有任何取代基的芳环的任何位置上选择性安装任何基团。然而,间-和对-C-H键的反应物控制的选择性功能化基本上是不可能的,因为它们本质上是矛盾的电子和空间需求。在这里,我们报告了催化剂的第一个例子,这些催化剂可以指导各种芳烃的可调硼化,并在间位或对位进行精确控制。位点通过配体的计算设计。范围广泛的氢键受体可以作为导向基团,包括 Weinreb 酰胺、膦酸盐和硼酸盐,它们可以很容易地转化为许多不同类型的官能团。这项工作还展示了过渡态计算在远程 C-H 活化催化剂设计中的关键作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号