Structure ( IF 4.4 ) Pub Date : 2022-05-23 , DOI: 10.1016/j.str.2022.04.014 Anna Czarna 1 , Jacek Plewka 1 , Leanid Kresik 1 , Alex Matsuda 1 , Abdulkarim Karim 2 , Colin Robinson 3 , Sean O'Byrne 3 , Fraser Cunningham 3 , Irene Georgiou 3 , Piotr Wilk 4 , Magdalena Pachota 1 , Grzegorz Popowicz 5 , Paul Graham Wyatt 3 , Grzegorz Dubin 6 , Krzysztof Pyrć 1
|
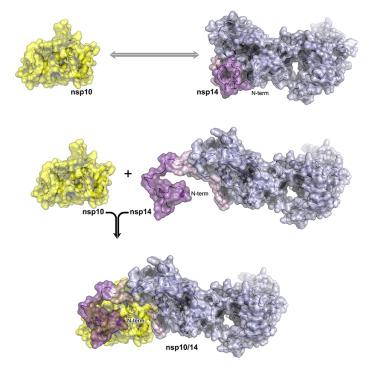
During RNA replication, coronaviruses require proofreading to maintain the integrity of their large genomes. Nsp14 associates with viral polymerase complex to excise the mismatched nucleotides. Aside from the exonuclease activity, nsp14 methyltransferase domain mediates cap methylation, facilitating translation initiation and protecting viral RNA from recognition by the innate immune sensors. The nsp14 exonuclease activity is modulated by a protein co-factor nsp10. While the nsp10/nsp14 complex structure is available, the mechanistic basis for nsp10-mediated modulation remains unclear in the absence of the nsp14 structure. Here, we provide a crystal structure of nsp14 in an apo-form. Comparative analysis of the apo- and nsp10-bound structures explain the modulatory role of the co-factor protein and reveal the allosteric nsp14 control mechanism essential for drug discovery. Further, the flexibility of the N-terminal lid of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) nsp14 structure presented in this study rationalizes the recently proposed idea of nsp14/nsp10/nsp16 ternary complex.
中文翻译:

nsp10 相互作用后 SARS-CoV-2 nsp14 的 cover 子结构域的重折叠释放核酸外切酶活性
在 RNA 复制过程中,冠状病毒需要进行校对以保持其大基因组的完整性。 Nsp14 与病毒聚合酶复合物结合以切除不匹配的核苷酸。除了核酸外切酶活性外,nsp14 甲基转移酶结构域还介导帽甲基化,促进翻译起始并保护病毒 RNA 免遭先天免疫传感器的识别。 nsp14 核酸外切酶活性由蛋白质辅因子 nsp10 调节。虽然 nsp10/nsp14 复合结构可用,但在缺乏 nsp14 结构的情况下,nsp10 介导的调节的机制基础仍不清楚。在这里,我们提供了 apo 形式的 nsp14 晶体结构。对 apo 和 nsp10 结合结构的比较分析解释了辅因子蛋白的调节作用,并揭示了药物发现所必需的变构 nsp14 控制机制。此外,本研究中提出的严重急性呼吸综合征冠状病毒 2 (SARS-CoV-2) nsp14 结构 N 端盖的灵活性合理化了最近提出的 nsp14/nsp10/nsp16 三元复合物的想法。







































 京公网安备 11010802027423号
京公网安备 11010802027423号