当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Metal-free dearomatization reactions of naphthol-ynamides for the divergent and enantioselective synthesis of azaspirocycles
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2022-05-23 , DOI: 10.1039/d2qo00685e Hang-Hao Li 1 , Yi-Ping Zhang 1 , Tong-Yi Zhai 1 , Bin-Yang Liu 1 , Chong-Yang Shi 1 , Jin-Mei Zhou 1 , Long-Wu Ye 1, 2
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2022-05-23 , DOI: 10.1039/d2qo00685e Hang-Hao Li 1 , Yi-Ping Zhang 1 , Tong-Yi Zhai 1 , Bin-Yang Liu 1 , Chong-Yang Shi 1 , Jin-Mei Zhou 1 , Long-Wu Ye 1, 2
Affiliation
|
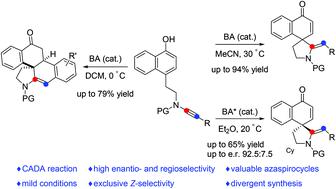
Dearomatization reactions of phenols and their derivatives have evolved as efficient and practical methods for the synthesis of functionalized spirocyclic enones in the past decades. Here, an efficient metal-free intramolecular dearomatization cyclization of readily available naphthol-ynamides has been developed, enabling practical, atom-economical and divergent access to two azaspirocyclic compounds in mostly good to excellent yields with a wide substrate scope. Moreover, such a catalytic asymmetric dearomatization reaction has also been achieved by employing a chiral Brønsted acid as a catalyst.
中文翻译:

萘酚酰胺的无金属脱芳构化反应用于氮杂螺环的发散和对映选择性合成
在过去的几十年中,酚类及其衍生物的脱芳构化反应已发展成为合成功能化螺环烯酮的有效且实用的方法。在这里,已经开发出一种有效的无金属分子内脱芳构环化现成的萘酚-炔酰胺,可以在广泛的底物范围内以大部分良好至优异的产率获得实用、原子经济和不同的两种氮杂螺环化合物。此外,这种催化不对称脱芳构化反应也已通过使用手性布朗斯台德酸作为催化剂来实现。
更新日期:2022-05-23
中文翻译:

萘酚酰胺的无金属脱芳构化反应用于氮杂螺环的发散和对映选择性合成
在过去的几十年中,酚类及其衍生物的脱芳构化反应已发展成为合成功能化螺环烯酮的有效且实用的方法。在这里,已经开发出一种有效的无金属分子内脱芳构环化现成的萘酚-炔酰胺,可以在广泛的底物范围内以大部分良好至优异的产率获得实用、原子经济和不同的两种氮杂螺环化合物。此外,这种催化不对称脱芳构化反应也已通过使用手性布朗斯台德酸作为催化剂来实现。











































 京公网安备 11010802027423号
京公网安备 11010802027423号