Journal of Catalysis ( IF 6.5 ) Pub Date : 2022-05-20 , DOI: 10.1016/j.jcat.2022.05.013 E. Zeynep Ayla , Darshan Patel , Arzam Harris , David W. Flaherty
|
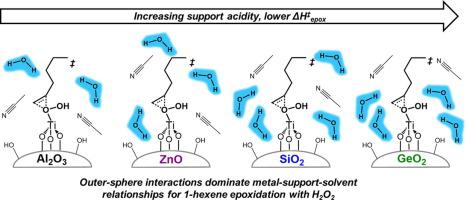
Atomically disperse Ti sites on metal oxides (MOx, including SiO2, γ-Al2O3, ZnO, GeO2) activate H2O2 to create intermediates active for alkene epoxidations. Turnover rates for 1-hexene epoxidation in acetonitrile vary 1000-fold at identical conditions due to differences in apparent activation enthalpies (ΔH‡epox) and entropies (ΔS‡epox). Ligand-to-metal charge transfer energies and vibrational frequencies of reactive species assessed by in situ UV–Vis and Raman spectroscopy, respectively, indicate supports do not detectably change electronic properties of H2O2-derived intermediates. However, isoelectric points and solution-phase water uptakes for these metal oxides correlate with ΔH‡epox and suggest that non-covalent interactions at the solid-liquid interface influence the stability of epoxidation transition states. Supports with lower pKa values concentrate water near the solid-liquid interface and enthalpically stabilize the transition state. These findings illustrate that outer sphere interactions impact epoxidation reactions upon metal oxide catalysts including titanium silicates.
中文翻译:

金属氧化物载体的特性控制着改变孤立钛原子上烯烃环氧化的速率和势垒的外球相互作用
原子分散在金属氧化物(MO x,包括 SiO 2、γ-Al 2 O 3、ZnO、GeO 2)上的 Ti 位点可激活 H 2 O 2以产生对烯烃环氧化具有活性的中间体。由于表观活化焓 (ΔH ‡ epox ) 和熵 (ΔS ‡ epox )的差异,在相同条件下,乙腈中 1-己烯环氧化的周转率变化 1000 倍。原位评估的活性物质的配体到金属的电荷转移能和振动频率UV-Vis 和拉曼光谱分别表明载体不会可检测地改变 H 2 O 2衍生中间体的电子特性。然而,这些金属氧化物的等电点和溶液相吸水量与 ΔH ‡ epox相关,表明固-液界面的非共价相互作用会影响环氧化过渡态的稳定性。以较低的 pK a值支持在固-液界面附近浓缩水并焓稳定过渡态。这些发现说明外球相互作用影响包括硅酸钛在内的金属氧化物催化剂的环氧化反应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号