当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Visible-Light-Promoted Radical Cyclization and N−N Bond Cleavage Relay of N-Aminopyridinium Ylides for Access to 2,3-Difunctionalized Indoles
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2022-05-20 , DOI: 10.1002/adsc.202200248 Meng-Meng Xu 1 , Wen-Bin Cao 1 , Xiaoping Xu 2 , Shun-Jun Ji 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2022-05-20 , DOI: 10.1002/adsc.202200248 Meng-Meng Xu 1 , Wen-Bin Cao 1 , Xiaoping Xu 2 , Shun-Jun Ji 1
Affiliation
|
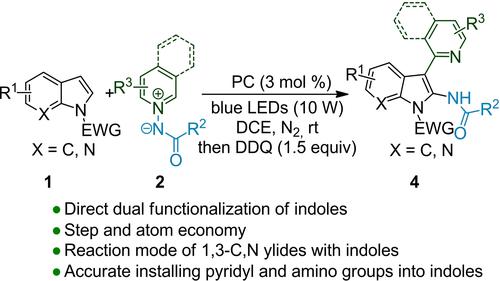
Reported herein is a photocatalytic platform for the vicinal aminopyridylation of indoles. The accurate difunctionalization reaction undergoes a tandem process involving a radical-mediated 1,3-dipolar cycloaddtion which generated from the single-electron oxidation of N-aminopyridinium ylides, a cleavage of the N−N bond as well as an oxidation of indoline ring. The electrophilic pyridyl and nucleophilic amino groups can be installed simultaneously into a wide range of indoles under mild and metal-free conditions. Moreover, the protocol shows high levels of step and atom economy.
中文翻译:

N-氨基吡啶鎓叶立德的可见光促进自由基环化和 N-N 键断裂中继以获得 2,3-双官能化吲哚
本文报道了一种用于吲哚连位氨基吡啶化的光催化平台。精确的双官能化反应经历了一个串联过程,涉及自由基介导的 1,3-偶极环加成反应,该反应由N-氨基吡啶鎓叶立德的单电子氧化、NN键的断裂以及二氢吲哚环的氧化产生。在温和和无金属条件下,亲电子吡啶基和亲核氨基可以同时安装到各种吲哚中。此外,该协议显示出高水平的步骤和原子经济性。
更新日期:2022-05-20
中文翻译:

N-氨基吡啶鎓叶立德的可见光促进自由基环化和 N-N 键断裂中继以获得 2,3-双官能化吲哚
本文报道了一种用于吲哚连位氨基吡啶化的光催化平台。精确的双官能化反应经历了一个串联过程,涉及自由基介导的 1,3-偶极环加成反应,该反应由N-氨基吡啶鎓叶立德的单电子氧化、NN键的断裂以及二氢吲哚环的氧化产生。在温和和无金属条件下,亲电子吡啶基和亲核氨基可以同时安装到各种吲哚中。此外,该协议显示出高水平的步骤和原子经济性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号