当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective Synthesis of the Chiral Pyrrolidine Fragment of Upadacitinib via Chiral Auxiliary Directed Diastereoselective 1,3-Dipolar Cycloaddition
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2022-05-20 , DOI: 10.1021/acs.oprd.1c00454 Magesh Sampath 1 , Sembian Ruso Jayaraman 1 , Vishnuvardhan Reddy Eda 1 , Rajendar Potham 1 , Rajeev Rehani Budhdev 2 , Saikat Sen 1 , Rakeshwar Bandichhor 2 , Srinivas Oruganti 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2022-05-20 , DOI: 10.1021/acs.oprd.1c00454 Magesh Sampath 1 , Sembian Ruso Jayaraman 1 , Vishnuvardhan Reddy Eda 1 , Rajendar Potham 1 , Rajeev Rehani Budhdev 2 , Saikat Sen 1 , Rakeshwar Bandichhor 2 , Srinivas Oruganti 1
Affiliation
|
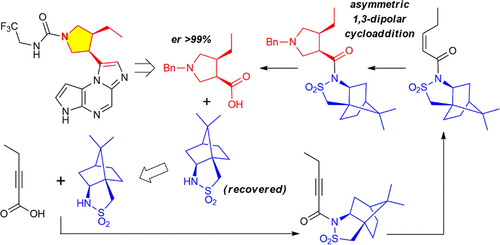
An efficient and elegant enantioselective synthesis of the key chiral pyrrolidine fragment of Upadacitinib (ABT-494) has been described. Oppolzer’s chiral sultam-directed asymmetric 1,3-dipolar cycloaddition was employed as a convenient tool to obtain the desired level of concomitant diastereoselectivity and enantioselectivity in the construction of the 3,4-syn substituted pyrrolidine moiety. The synthesis process was demonstrated as a proof of study on a lab scale and was refined during scale-up to allow for easy disengagement of the chiral auxiliary and its subsequent reuse.
中文翻译:

通过手性辅助定向非对映选择性 1,3-偶极环加成对映选择性合成 Upadacitinib 的手性吡咯烷片段
已经描述了 Upadacitinib (ABT-494) 的关键手性吡咯烷片段的高效和优雅的对映选择性合成。Oppolzer 的手性苏丹定向不对称 1,3-偶极环加成被用作在构建 3,4-顺式取代的吡咯烷部分中获得所需水平的伴随非对映选择性和对映选择性的便利工具。该合成过程被证明是实验室规模的研究证明,并在放大过程中进行了改进,以使手性助剂易于脱离并随后重复使用。
更新日期:2022-05-20
中文翻译:

通过手性辅助定向非对映选择性 1,3-偶极环加成对映选择性合成 Upadacitinib 的手性吡咯烷片段
已经描述了 Upadacitinib (ABT-494) 的关键手性吡咯烷片段的高效和优雅的对映选择性合成。Oppolzer 的手性苏丹定向不对称 1,3-偶极环加成被用作在构建 3,4-顺式取代的吡咯烷部分中获得所需水平的伴随非对映选择性和对映选择性的便利工具。该合成过程被证明是实验室规模的研究证明,并在放大过程中进行了改进,以使手性助剂易于脱离并随后重复使用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号