当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Direct Thioamination of Cyclohexanones via Difunctionalization with Thiophenol and Aniline
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2022-05-19 , DOI: 10.1002/adsc.202200365 Minli Tang 1 , Li Zhang 2 , Guojiang Mao 3 , Fuhong Xiao 4 , Wen Shao 1 , Guojun Deng 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2022-05-19 , DOI: 10.1002/adsc.202200365 Minli Tang 1 , Li Zhang 2 , Guojiang Mao 3 , Fuhong Xiao 4 , Wen Shao 1 , Guojun Deng 1
Affiliation
|
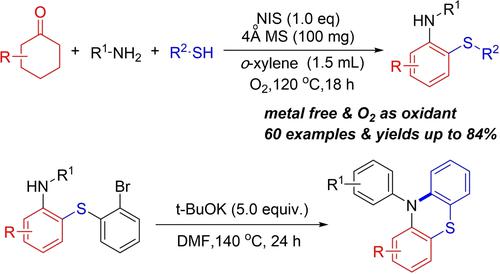
A synthetic method of o-sulfanylanilines from cyclohexanones, amines, and thiophenols under transition-metal-free conditions is disclosed. This is the first report on the direct thioamination of cyclohexanones via N-iodosuccinimide (NIS) promoted oxidative dehydroaromatization. Non-aromatic cyclohexanones were smoothly dehydrogenated, and acted as an aryl source using oxygen as a green oxidant. The capacity of the resultant o-sulfanylanilines for synthesis of N-arylphenothiazines has been further demonstrated.
中文翻译:

用苯硫酚和苯胺双官能化环己酮直接硫胺化
公开了一种在无过渡金属条件下由环己酮、胺和苯硫酚合成邻硫基苯胺的方法。这是关于通过N-碘代琥珀酰亚胺 (NIS) 促进氧化脱氢芳构化环己酮直接硫胺化的第一份报告。非芳香环己酮被顺利脱氢,并使用氧作为绿色氧化剂作为芳基源。所得邻硫基苯胺用于合成N-芳基吩噻嗪的能力已得到进一步证明。
更新日期:2022-05-19
中文翻译:

用苯硫酚和苯胺双官能化环己酮直接硫胺化
公开了一种在无过渡金属条件下由环己酮、胺和苯硫酚合成邻硫基苯胺的方法。这是关于通过N-碘代琥珀酰亚胺 (NIS) 促进氧化脱氢芳构化环己酮直接硫胺化的第一份报告。非芳香环己酮被顺利脱氢,并使用氧作为绿色氧化剂作为芳基源。所得邻硫基苯胺用于合成N-芳基吩噻嗪的能力已得到进一步证明。









































 京公网安备 11010802027423号
京公网安备 11010802027423号