Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy ( IF 4.3 ) Pub Date : 2022-05-18 , DOI: 10.1016/j.saa.2022.121400 Walaa H El-Shwiniy 1 , Sameh I El-Desoky 2 , Ali Alrabie 3 , Badr Abd El-Wahaab 4
|
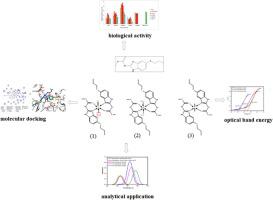
Spectrophotometry was used to determine trace amounts of Zirconium(IV), Mercury(II) and Uranium(VI) in environmental, biological, pharmaceutical and industrial samples. The determination depend on the complexation reactions between albendazole reagent and metal ions [Zr(IV), Hg(II) and U(VI)] at 555 nm, 485 nm and 510 nm, respectively. The experimental conditions were explored to reach the optimum conditions for albendazole-metal ions interaction, including detection of a suitable wavelength, medium (pH), reagent concentration, surfactants effect, reaction time and temperature. Under optimum conditions, the complexes displayed apparent molar absorptivities of 0.8350 × 104, 0.6210 × 104 and 0.7012 × 104 L mol−1 cm−1; Sandell’s sensitivity of 0.01092, 0.03230 and 0.03394 µg cm−2 and with linearity ranges of 1.0–120.0, 3.0–200.0 and 1.0–150.0 µg mL−1 for the developed methods, respectively. Furthermore, Elemental analysis, thermal analysis (TGA, DTG), IR, 1HNMR, spectroscopies, electrical molar conductivity and magnetic moment measurements were used to determine the structures and characteristics of the complexes. A careful examination of the IR spectra revealed that the ligand interacted with all of the metal ions described as a bidentate via the oxygen of the carbonyl of the ester moiety and the nitrogen atom of the heterocyclic  C
C N
N group. An octahedral geometry for Zr(IV), Hg(II) and U(VI) complexes has been postulated based on magnetic and electronic spectrum data. The band gap values indicated that these complexes were semi-conductors and belong to the same class of extremely effective solar materials. The albendazole ligand and its complexes have been biologically tested against a variety of bacterial and fungal strains, and molecular docking studies have been conducted to evaluate the optimal binding site and its inhibitory action.
group. An octahedral geometry for Zr(IV), Hg(II) and U(VI) complexes has been postulated based on magnetic and electronic spectrum data. The band gap values indicated that these complexes were semi-conductors and belong to the same class of extremely effective solar materials. The albendazole ligand and its complexes have been biologically tested against a variety of bacterial and fungal strains, and molecular docking studies have been conducted to evaluate the optimal binding site and its inhibitory action.
中文翻译:

溶液中 Zr(IV)、Hg(II) 和 U(VI) 的分光光度测定及其分析应用:固体配合物的结构表征和分子对接
分光光度法用于测定环境、生物、制药和工业样品中的痕量锆 (IV)、汞 (II) 和铀 (VI)。该测定取决于阿苯达唑试剂和金属离子 [Zr(IV)、Hg(II) 和 U(VI)] 分别在 555 nm、485 nm 和 510 nm 处的络合反应。探索了实验条件,以达到阿苯达唑-金属离子相互作用的最佳条件,包括检测合适的波长、介质(pH)、试剂浓度、表面活性剂效应、反应时间和温度。在最佳条件下,配合物的表观摩尔吸光率为0.8350 × 10 4、0.6210 × 10 4和0.7012 × 10 4 L mol -1 cm -1; 对于所开发的方法, Sandell 的灵敏度分别为 0.01092、0.03230 和 0.03394 µg cm -2,线性范围分别为 1.0–120.0、3.0–200.0 和 1.0–150.0 µg mL -1。此外,元素分析、热分析(TGA、DTG)、IR、1 HNMR、光谱、摩尔电导率和磁矩测量用于确定配合物的结构和特性。对红外光谱的仔细检查表明,配体通过酯部分羰基的氧和杂环 C
C  N的氮原子与所有描述为双齿的金属离子相互作用
N的氮原子与所有描述为双齿的金属离子相互作用 团体。Zr(IV)、Hg(II) 和 U(VI) 配合物的八面体几何形状已根据磁性和电子光谱数据进行了假设。带隙值表明这些配合物是半导体,属于同一类极其有效的太阳能材料。阿苯达唑配体及其复合物已针对多种细菌和真菌菌株进行了生物学测试,并进行了分子对接研究以评估最佳结合位点及其抑制作用。
团体。Zr(IV)、Hg(II) 和 U(VI) 配合物的八面体几何形状已根据磁性和电子光谱数据进行了假设。带隙值表明这些配合物是半导体,属于同一类极其有效的太阳能材料。阿苯达唑配体及其复合物已针对多种细菌和真菌菌株进行了生物学测试,并进行了分子对接研究以评估最佳结合位点及其抑制作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号