当前位置:
X-MOL 学术
›
Mol. Microbiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure-function analysis for the development of peptide inhibitors for a Gram-positive quorum sensing system
Molecular Microbiology ( IF 2.6 ) Pub Date : 2022-05-16 , DOI: 10.1111/mmi.14921 Iman Tajer Abdullah 1, 2 , Andrew T Ulijasz 3 , Umakhanth Venkatraman Girija 1 , Sien Tam 4 , Peter Andrew 1 , Natalia Luisa Hiller 4 , Russell Wallis 1 , Hasan Yesilkaya 1
Molecular Microbiology ( IF 2.6 ) Pub Date : 2022-05-16 , DOI: 10.1111/mmi.14921 Iman Tajer Abdullah 1, 2 , Andrew T Ulijasz 3 , Umakhanth Venkatraman Girija 1 , Sien Tam 4 , Peter Andrew 1 , Natalia Luisa Hiller 4 , Russell Wallis 1 , Hasan Yesilkaya 1
Affiliation
|
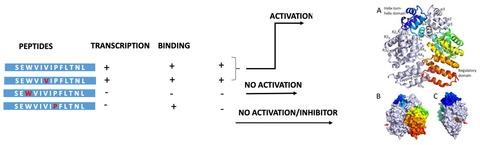
The Streptococcus pneumoniae Rgg144/SHP144 regulator-peptide quorum sensing (QS) system is critical for nutrient utilization, oxidative stress response, and virulence. Here, we characterized this system by assessing the importance of each residue within the active short hydrophobic peptide (SHP) by alanine-scanning mutagenesis and testing the resulting peptides for receptor binding and activation of the receptor. Interestingly, several of the mutations had little effect on binding to Rgg144 but reduced transcriptional activation appreciably. In particular, a proline substitution (P21A) reduced transcriptional activation by 29-fold but bound with a 3-fold higher affinity than the wild-type SHP. Consistent with the function of Rgg144, the mutant peptide led to decreased utilization of mannose and increased susceptibility to superoxide generator paraquat. Pangenome comparison showed full conservation of P21 across SHP144 allelic variants. Crystallization of Rgg144 in the absence of peptide revealed a comparable structure to the DNA bound and free forms of its homologs suggesting similar mechanisms of activation. Together, these analyses identify key interactions in a critical pneumococcal QS system. Further manipulation of the SHP has the potential to facilitate the development of inhibitors that are functional across strains. The approach described here is likely to be effective across QS systems in multiple species.
中文翻译:

革兰氏阳性群体感应系统肽抑制剂开发的结构功能分析
肺炎链球菌Rgg144/SHP144 调节肽群体感应 (QS) 系统对于养分利用、氧化应激反应和毒力至关重要。在这里,我们通过丙氨酸扫描诱变评估活性短疏水肽 (SHP) 中每个残基的重要性并测试所得肽的受体结合和受体活化来表征该系统。有趣的是,一些突变对与 Rgg144 的结合几乎没有影响,但显着降低了转录激活。特别是,脯氨酸取代 (P21A) 将转录激活减少了 29 倍,但结合的亲和力比野生型 SHP 高 3 倍。与 Rgg144 的功能一致,突变肽导致甘露糖利用率降低,对超氧化物发生器百草枯的敏感性增加。Pangenome 比较显示 P21 在 SHP144 等位基因变体中完全保守。在不存在肽的情况下,Rgg144 的结晶揭示了与其同源物的 DNA 结合和游离形式相当的结构,表明了类似的激活机制。总之,这些分析确定了关键肺炎球菌 QS 系统中的关键相互作用。对 SHP 的进一步操作有可能促进跨菌株发挥功能的抑制剂的开发。此处描述的方法可能对多个物种的 QS 系统有效。这些分析确定了关键肺炎球菌 QS 系统中的关键相互作用。对 SHP 的进一步操作有可能促进跨菌株发挥功能的抑制剂的开发。此处描述的方法可能对多个物种的 QS 系统有效。这些分析确定了关键肺炎球菌 QS 系统中的关键相互作用。对 SHP 的进一步操作有可能促进跨菌株发挥功能的抑制剂的开发。此处描述的方法可能对多个物种的 QS 系统有效。
更新日期:2022-05-16
中文翻译:

革兰氏阳性群体感应系统肽抑制剂开发的结构功能分析
肺炎链球菌Rgg144/SHP144 调节肽群体感应 (QS) 系统对于养分利用、氧化应激反应和毒力至关重要。在这里,我们通过丙氨酸扫描诱变评估活性短疏水肽 (SHP) 中每个残基的重要性并测试所得肽的受体结合和受体活化来表征该系统。有趣的是,一些突变对与 Rgg144 的结合几乎没有影响,但显着降低了转录激活。特别是,脯氨酸取代 (P21A) 将转录激活减少了 29 倍,但结合的亲和力比野生型 SHP 高 3 倍。与 Rgg144 的功能一致,突变肽导致甘露糖利用率降低,对超氧化物发生器百草枯的敏感性增加。Pangenome 比较显示 P21 在 SHP144 等位基因变体中完全保守。在不存在肽的情况下,Rgg144 的结晶揭示了与其同源物的 DNA 结合和游离形式相当的结构,表明了类似的激活机制。总之,这些分析确定了关键肺炎球菌 QS 系统中的关键相互作用。对 SHP 的进一步操作有可能促进跨菌株发挥功能的抑制剂的开发。此处描述的方法可能对多个物种的 QS 系统有效。这些分析确定了关键肺炎球菌 QS 系统中的关键相互作用。对 SHP 的进一步操作有可能促进跨菌株发挥功能的抑制剂的开发。此处描述的方法可能对多个物种的 QS 系统有效。这些分析确定了关键肺炎球菌 QS 系统中的关键相互作用。对 SHP 的进一步操作有可能促进跨菌株发挥功能的抑制剂的开发。此处描述的方法可能对多个物种的 QS 系统有效。











































 京公网安备 11010802027423号
京公网安备 11010802027423号