当前位置:
X-MOL 学术
›
Mol. Microbiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Streptococcus pneumoniae binds collagens and C1q via the SSURE repeats of the PfbB adhesin
Molecular Microbiology ( IF 2.6 ) Pub Date : 2022-05-15 , DOI: 10.1111/mmi.14920 Giuseppe Valerio De Gaetano 1 , Francesco Coppolino 2 , Germana Lentini 1 , Agata Famà 1 , Chiara Cullotta 1 , Ivana Raffaele 1 , Chiara Motta 3 , Giuseppe Teti 4 , Pietro Speziale 3 , Giampiero Pietrocola 3 , Concetta Beninati 1, 5
Molecular Microbiology ( IF 2.6 ) Pub Date : 2022-05-15 , DOI: 10.1111/mmi.14920 Giuseppe Valerio De Gaetano 1 , Francesco Coppolino 2 , Germana Lentini 1 , Agata Famà 1 , Chiara Cullotta 1 , Ivana Raffaele 1 , Chiara Motta 3 , Giuseppe Teti 4 , Pietro Speziale 3 , Giampiero Pietrocola 3 , Concetta Beninati 1, 5
Affiliation
|
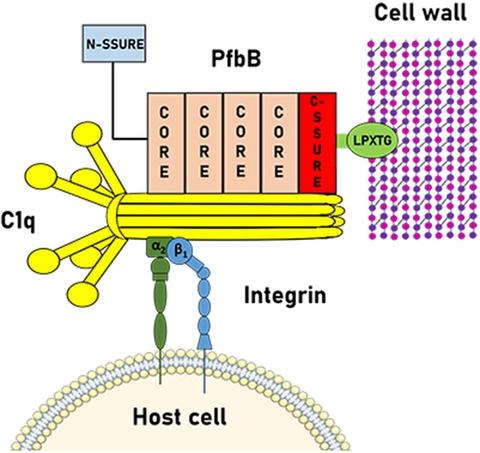
The binding of Streptococcus pneumoniae to collagen is likely an important step in the pathogenesis of pneumococcal infections, but little is known of the underlying molecular mechanisms. Streptococcal surface repeats (SSURE) are highly conserved protein domains present in cell wall adhesins from different Streptococcus species. We find here that SSURE repeats of the pneumococcal adhesin plasminogen and fibronectin binding protein B (PfbB) bind to various types of collagen. Moreover, deletion of the pfbB gene resulted in a significant impairment of the ability of encapsulated or unencapsulated pneumococci to bind collagen. Notably, a PfbB SSURE domain is also bound to the complement component C1q that bears a collagen-like domain and promotes adherence of pneumococci to host cells by acting as a bridge between bacteria and epithelial cells. Accordingly, deletion of PfbB or pre-treatment with anti-SSURE antibodies markedly decreased pneumococcal binding to C1q as well as C1q-dependent adherence to epithelial and endothelial cells. Further data indicated that C1q promotes pneumococcal adherence by binding to integrin α2β1. In conclusion, our results indicate that the SSURE domains of the PfbB protein promote interactions of pneumococci with various types of collagen and with C1q. These repeats may be useful targets in strategies to control S. pneumoniae infections.
中文翻译:

肺炎链球菌通过 PfbB 粘附素的 SSURE 重复序列结合胶原蛋白和 C1q
肺炎链球菌与胶原蛋白的结合可能是肺炎球菌感染发病机制中的一个重要步骤,但对其潜在的分子机制知之甚少。链球菌表面重复( SSURE ) 是高度保守的蛋白质结构域,存在于来自不同链球菌物种的细胞壁粘附素中。我们在这里发现肺炎球菌粘附素纤溶酶原和纤连蛋白结合蛋白B ( PfbB )的 SSURE 重复序列与各种类型的胶原蛋白结合。此外,删除pfbB基因导致封装或未封装的肺炎球菌结合胶原蛋白的能力显着受损。值得注意的是,PfbB SSURE 结构域也与补体成分 C1q 结合,该补体成分 C1q 具有胶原样结构域,并通过充当细菌和上皮细胞之间的桥梁来促进肺炎球菌与宿主细胞的粘附。因此,PfbB 的缺失或用抗 SSURE 抗体预处理显着降低了肺炎球菌与 C1q 的结合以及 C1q 依赖性对上皮细胞和内皮细胞的粘附。进一步的数据表明,C1q 通过与整合素 α 2 β 1结合来促进肺炎球菌的粘附. 总之,我们的结果表明 PfbB 蛋白的 SSURE 结构域促进肺炎球菌与各种类型的胶原蛋白和 C1q 的相互作用。这些重复可能是控制肺炎链球菌感染策略中的有用目标。
更新日期:2022-05-15
中文翻译:

肺炎链球菌通过 PfbB 粘附素的 SSURE 重复序列结合胶原蛋白和 C1q
肺炎链球菌与胶原蛋白的结合可能是肺炎球菌感染发病机制中的一个重要步骤,但对其潜在的分子机制知之甚少。链球菌表面重复( SSURE ) 是高度保守的蛋白质结构域,存在于来自不同链球菌物种的细胞壁粘附素中。我们在这里发现肺炎球菌粘附素纤溶酶原和纤连蛋白结合蛋白B ( PfbB )的 SSURE 重复序列与各种类型的胶原蛋白结合。此外,删除pfbB基因导致封装或未封装的肺炎球菌结合胶原蛋白的能力显着受损。值得注意的是,PfbB SSURE 结构域也与补体成分 C1q 结合,该补体成分 C1q 具有胶原样结构域,并通过充当细菌和上皮细胞之间的桥梁来促进肺炎球菌与宿主细胞的粘附。因此,PfbB 的缺失或用抗 SSURE 抗体预处理显着降低了肺炎球菌与 C1q 的结合以及 C1q 依赖性对上皮细胞和内皮细胞的粘附。进一步的数据表明,C1q 通过与整合素 α 2 β 1结合来促进肺炎球菌的粘附. 总之,我们的结果表明 PfbB 蛋白的 SSURE 结构域促进肺炎球菌与各种类型的胶原蛋白和 C1q 的相互作用。这些重复可能是控制肺炎链球菌感染策略中的有用目标。











































 京公网安备 11010802027423号
京公网安备 11010802027423号