Structure ( IF 4.4 ) Pub Date : 2022-05-16 , DOI: 10.1016/j.str.2022.04.011 Jiemin Shen 1 , Miaohui Hu 2 , Xiao Fan 2 , Zhenning Ren 1 , Corinne Portioli 1 , Xiuwen Yan 1 , Mingqiang Rong 1 , Ming Zhou 1
|
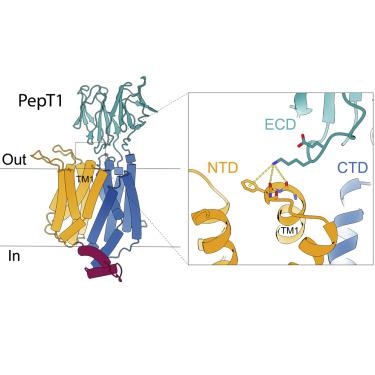
Mammalian peptide transporters, PepT1 and PepT2, mediate uptake of small peptides and are essential for their absorption. PepT also mediates absorption of many drugs and prodrugs to enhance their bioavailability. PepT has twelve transmembrane (TM) helices that fold into an N-terminal domain (NTD, TM1-6) and a C-terminal domain (CTD, TM7-12) and has a large extracellular domain (ECD) between TM9-10. It is well recognized that peptide transport requires movements of the NTD and CTD, but the role of the ECD in PepT1 remains unclear. Here we report the structure of horse PepT1 encircled in lipid nanodiscs and captured in the inward-open apo conformation. The structure shows that the ECD bridges the NTD and CTD by interacting with TM1. Deletion of ECD or mutations to the ECD-TM1 interface impairs the transport activity. These results demonstrate an important role of ECD in PepT1 and enhance our understanding of the transport mechanism in PepT1.
中文翻译:

PepT1 的胞外域与 TM1 相互作用以促进底物运输
哺乳动物肽转运蛋白 PepT1 和 PepT2 介导小肽的摄取,对于小肽的吸收至关重要。PepT 还介导许多药物和前药的吸收,以提高其生物利用度。PepT 具有 12 个跨膜 (TM) 螺旋,可折叠成 N 端结构域(NTD、TM1-6)和 C 端结构域(CTD、TM7-12),并在 TM9-10 之间具有较大的胞外结构域 (ECD)。众所周知,肽转运需要 NTD 和 CTD 的运动,但 ECD 在 PepT1 中的作用仍不清楚。在这里,我们报道了马 PepT1 的结构,该结构被脂质纳米盘包围并以向内开放的 apo 构象捕获。该结构表明 ECD 通过与 TM1 相互作用来桥接 NTD 和 CTD。ECD 的缺失或 ECD-TM1 接口的突变会损害转运活性。这些结果证明了 ECD 在 PepT1 中的重要作用,并增强了我们对 PepT1 转运机制的理解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号