Structure ( IF 4.4 ) Pub Date : 2022-05-16 , DOI: 10.1016/j.str.2022.04.010 Zephan Melville 1 , Haikel Dridi 1 , Qi Yuan 1 , Steven Reiken 1 , Anetta Wronska 1 , Yang Liu 1 , Oliver B Clarke 2 , Andrew R Marks 3
|
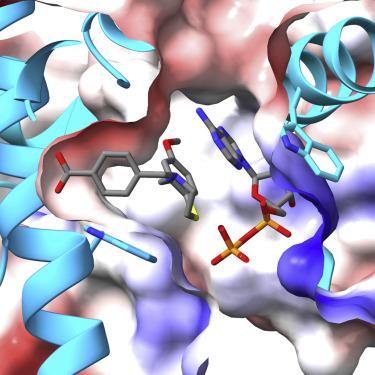
The ryanodine receptor (RyR)/calcium release channel on the sarcoplasmic reticulum (SR) is required for excitation-contraction coupling in skeletal and cardiac muscle. Inherited mutations and stress-induced post-translational modifications result in an SR Ca2+ leak that causes skeletal myopathies, heart failure, and exercise-induced sudden death. A class of therapeutics known as Rycals prevent the RyR-mediated leak, are effective in preventing disease progression and restoring function in animal models, and are in clinical trials for patients with muscle and heart disorders. Using cryogenic-electron microscopy, we present a model of RyR1 with a 2.45-Å resolution before local refinement, revealing a binding site in the RY1&2 domain (3.10 Å local resolution), where the Rycal ARM210 binds cooperatively with ATP and stabilizes the closed state of RyR1.
中文翻译:

1 型兰尼碱受体中的药物和 ATP 结合位点
肌浆网 (SR) 上的兰尼碱受体 (RyR)/钙释放通道是骨骼肌和心肌中兴奋-收缩耦合所必需的。遗传突变和应激诱导的翻译后修饰会导致 SR Ca 2+渗漏,从而导致骨骼肌病、心力衰竭和运动诱发的猝死。一类名为 Rycals 的疗法可预防 RyR 介导的渗漏,可有效预防疾病进展并恢复动物模型的功能,并且正在针对肌肉和心脏疾病患者进行临床试验。使用低温电子显微镜,我们展示了局部细化前分辨率为 2.45 Å 的 RyR1 模型,揭示了 RY1&2 结构域中的结合位点(局部分辨率为 3.10 Å),其中 Rycal ARM210 与 ATP 协同结合并稳定闭合状态RyR1。











































 京公网安备 11010802027423号
京公网安备 11010802027423号