Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy ( IF 4.3 ) Pub Date : 2022-05-16 , DOI: 10.1016/j.saa.2022.121395 Wen Juan Liang 1 , Wen Xin Wu 2 , Zhen Lu 1 , Yun Feng Bai 1 , Feng Feng 1 , Wei Jun Jin 3
|
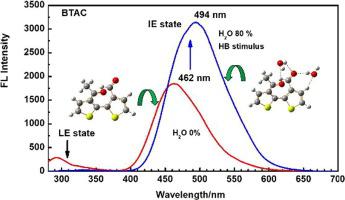
Two dithiophene aldehyde/ketone derivatives are designed as luminescence molecular rotors, i.e., 1,1′-([2,2′-bithiophene]-3,3′-diyl)bis(ethan-1-one) (BTBE) and 3′-acetyl-[2,2′-bithiophene]-3-carbaldehyde (BTAC). Their absorption and luminescence properties, as well as the stimulus responsive luminescence characteristics of water spikes are studied in detail. In order to further explore relationship of luminescence and molecular structure, three reference compounds are also synthesized, named 1-(2-methylthiophen-3-yl)ethanone (MTE), 2-methylthiophene-3-carbaldehyde (MTC) and 4H-cyclohepta[1,2-b:7,6-b’]dithiophen-4-one (CDTO). BTBE and BTAC have two obvious absorption bands in the short wavelength region with peak wavelengths of about 212 nm and 260 nm, respectively, while there is a weak trailing type absorption band in the range of about 300–400 nm. Their fluorescence spectra show two luminescence bands in the range of 280–350 nm and 400–600 nm, respectively, and the latter is stronger. Compared with the absorption and luminescence spectra of the reference compounds, it is determined that two absorption bands of BTBE and BTAC in shorter wavelength region are derived from the single thiophene ring carbonyl planar unit, while the absorption band in longer region are derived from the integrated structure of dithiophene carbonyl. The fluorescence bands with peaks of about 300 nm and 470 nm originate respectively from the localized F-C vertical excited states (LE), i.e., the excited state from single planar thienyl-carbonyl unit, and integrated excited state (IE), i.e., the excited state from integrated di-thienyl-carbonyl rings linked covalently with less dihedral angle and greater degree of conjugation at excited state. The crystal structure data show that two thiophene rings possess larger dihedral angles in crystal states, 86.9° for BTBE and 60.8° for BTAC, respectively. However, theoretical calculation results prove the conformational stabilization energy changes little, less than 1.5 kcal/mol, as dihedral angle changes from 50° to 100°. Hydrogen bonding is sufficient to overcome the energy required for this conformational change. Therefore, both BTBE and BTAC can produce water stimulation response luminescence behavior. This stimulating response behavior of BTBE and BTAC can be applied to the preparation of water writable film materials.
中文翻译:

1,1'-([2,2'-联噻吩]-3,3'-二基)双(ethan-1-one)和3'-乙酰基-[2,2'-转子型的刺激响应发光及应用二噻吩]-3-甲醛作为分子转子
设计了两种二噻吩醛/酮衍生物作为发光分子转子,即1,1'-([2,2'-bithiophene]-3,3'-diyl)bis(ethan-1-one) ( BTBE ) 和3'-乙酰基-[2,2'-联噻吩]-3-甲醛(BTAC)。详细研究了它们的吸收和发光特性,以及水尖峰的刺激响应发光特性。为了进一步探索发光与分子结构的关系,还合成了三种参考化合物,分别命名为 1-(2-methylthiophen-3-yl)ethanone ( MTE )、2-methylthiophene-3-carbaldehyde ( MTC ) 和 4H-cyclohepta [1,2-b:7,6-b']dithiophen-4-one ( CDTO )。BTBE和BTAC在短波长区域有两个明显的吸收带,峰值波长分别约为 212 nm 和 260 nm,而在 300-400 nm 范围内有一个微弱的拖尾型吸收带。它们的荧光光谱分别在 280-350 nm 和 400-600 nm 范围内显示出两个发光带,后者更强。与参比化合物的吸收光谱和发光光谱比较,确定了BTBE和BTAC的两个吸收带较短波长区域的吸收带来源于单个噻吩环羰基平面单元,而较长区域的吸收带来源于二噻吩羰基的整体结构。峰约300 nm和470 nm的荧光带分别来源于局域FC垂直激发态(LE),即单一平面噻吩基-羰基单元的激发态,和积分激发态(IE),即激发态由集成的二噻吩基羰基环以较小的二面角和更大程度的激发态共价共价连接的状态。晶体结构数据表明,两个噻吩环在晶态下具有较大的二面角,BTBE为86.9°, BTAC为60.8°, 分别。然而,理论计算结果证明,随着二面角从 50° 变为 100°,构象稳定能变化很小,小于 1.5 kcal/mol。氢键足以克服这种构象变化所需的能量。因此,BTBE和BTAC都可以产生水刺激响应发光行为。BTBE和BTAC的这种刺激反应行为可以应用于水可写薄膜材料的制备。











































 京公网安备 11010802027423号
京公网安备 11010802027423号