当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Palladium-catalyzed [4 + 4] cycloadditions for highly diastereo- and enantioselective synthesis of functionalized benzo[b]oxocines
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2022-05-16 , DOI: 10.1039/d2qo00422d Xunhua Wang 1 , Jianfeng Yang 1 , Ruifeng Lv 1 , Pengfei Song 1 , Denghui Ye 1 , Jitian Liu 1 , Xiaoxun Li 1
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2022-05-16 , DOI: 10.1039/d2qo00422d Xunhua Wang 1 , Jianfeng Yang 1 , Ruifeng Lv 1 , Pengfei Song 1 , Denghui Ye 1 , Jitian Liu 1 , Xiaoxun Li 1
Affiliation
|
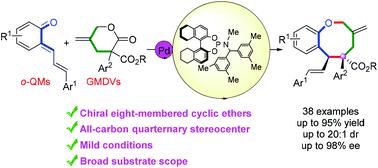
Asymmetric cycloaddition reactions represent a powerful strategy for building up complex molecular architectures, especially those with medium-sized rings. Herein, we disclose a highly diastereo- and enantioselective cycloaddition strategy that involves the palladium-catalyzed [4 + 4] cycloaddition between ortho-quinone methides and γ-methylene-δ-valerolactones. This method provided a straightforward and applicable approach to access various functionalized benzo[b]oxocines bearing adjacent all-carbon quaternary and tertiary stereocenters (38 examples, up to 95% yield, 20 : 1 dr, 98% ee). The process was efficient and scalable, and the products could be further transformed to various chiral eight-membered molecules. In addition, DFT calculations were performed to shed light on the mechanism of good stereoselectivities.
中文翻译:

钯催化的 [4 + 4] 环加成用于高度非对映和对映选择性合成官能化苯并 [b] 氧杂辛
不对称环加成反应代表了构建复杂分子结构的强大策略,特别是那些具有中等大小环的分子结构。在此,我们公开了一种高度非对映和对映选择性的环加成策略,该策略涉及邻苯醌甲基化物和 γ-亚甲基-δ-戊内酯之间的钯催化的 [4 + 4] 环加成反应。该方法提供了一种直接且适用的方法来获取各种功能化苯并[ b]oxocines 具有相邻的全碳四级和三级立体中心(38 个示例,产率高达 95%,20:1 dr,98% ee)。该过程高效且可扩展,产品可以进一步转化为各种手性八元分子。此外,还进行了 DFT 计算以阐明良好立体选择性的机制。
更新日期:2022-05-19
中文翻译:

钯催化的 [4 + 4] 环加成用于高度非对映和对映选择性合成官能化苯并 [b] 氧杂辛
不对称环加成反应代表了构建复杂分子结构的强大策略,特别是那些具有中等大小环的分子结构。在此,我们公开了一种高度非对映和对映选择性的环加成策略,该策略涉及邻苯醌甲基化物和 γ-亚甲基-δ-戊内酯之间的钯催化的 [4 + 4] 环加成反应。该方法提供了一种直接且适用的方法来获取各种功能化苯并[ b]oxocines 具有相邻的全碳四级和三级立体中心(38 个示例,产率高达 95%,20:1 dr,98% ee)。该过程高效且可扩展,产品可以进一步转化为各种手性八元分子。此外,还进行了 DFT 计算以阐明良好立体选择性的机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号