Soil Biology and Biochemistry ( IF 9.7 ) Pub Date : 2022-05-14 , DOI: 10.1016/j.soilbio.2022.108711 Yunying Fang , Ehsan Tavakkoli , Zhe Weng , Damian Collins , Deirdre Harvey , Niloofar Karimian , Yu Luo , Promil Mehra , Michael T. Rose , Nigel Wilhelm , Lukas Van Zwieten
|
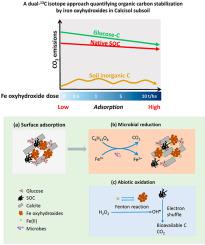
Calcisols pose some unique challenges, particularly relating to their low organic carbon (C) content and low C storage ceiling. To address this, we investigated the role of iron (Fe) oxyhydroxides – goethite and ferrihydrite (0.36, 0.72, 3.6, and 7.2 g kg−1 soil) in the presence of a labile C substrate (glucose) to simulate rhizodeposition, on C-cycling. As there were three potential C sources: (i) glucose-C, (ii) native SOC, and (iii) soil inorganic C (SIC), a novel dual-13C isotope approach (δ13C-enriched glucose of 29 and 81‰) was implemented to accurately differentiate these three C sources from a Calcisol subsoil (δ13SOC, −23‰; δ13SIC, −3.6‰). Over 28 days, across the glucose and Fe oxyhydroxide treatments, 34.8–41.7% of the supplied glucose-C (1.0 g C kg−1 soil), 7.5–9.6% of the native SOC (3.7 g kg−1 soil), and 0.11–0.19% of the SIC (48 g kg−1 soil) were lost as CO2. Goethite and ferrihydrite generally stabilized organic C (including glucose-C and native SOC) which occurred primarily within the first 10 days following amendment with Fe oxyhydroxide, and the stabilization effect generally increased with increasing Fe oxyhydroxide dose. This is likely due to rapid Fe-OC adsorption that protected the OC from microbial decomposition. Ferrihydrite (cf. goethite) had a smaller effect on suppressing positive priming of SOC mineralization induced by glucose, possibly resulting from the lower C use efficiency and less stable Fe-OC associations due to the higher dissolution rate of ferrihydrite. The SIC loss increased after glucose addition, which was further enhanced by Fe oxyhydroxides. We conclude that Fe oxyhydroxides may be useful amendments for increasing SOC in highly alkaline Calcisols.
中文翻译:

在用羟基氧化铁修正的钙溶胶底土中解开碳稳定:双 13C 同位素方法
钙化溶胶带来了一些独特的挑战,特别是与它们的低有机碳 (C) 含量和低 C 储存上限有关。为了解决这个问题,我们研究了铁 (Fe) 羟基氧化物 - 针铁矿和水铁矿(0.36、0.72、3.6 和 7.2 g kg -1土壤)在不稳定的 C 底物(葡萄糖)存在下的作用,以模拟 C 上的根际沉积。 -骑自行车。由于存在三种潜在的 C 源:(i) 葡萄糖-C、(ii) 天然 SOC 和 (iii) 土壤无机碳 (SIC),这是一种新的双13 C 同位素方法(富含δ 13 C 的葡萄糖 29 和81‰)用于准确区分这三种碳源与钙溶胶底土(δ 13 SOC,-23‰;δ 13SIC,-3.6‰)。超过 28 天,在葡萄糖和羟基氧化铁处理中,34.8-41.7% 的供应葡萄糖-C(1.0 g C kg -1土壤),7.5-9.6% 的天然 SOC(3.7 g kg -1土壤),和0.11-0.19% 的 SIC(48 g kg -1土壤)以 CO 2形式流失。针铁矿和水铁矿一般稳定有机碳(包括葡萄糖碳和天然 SOC),主要发生在用羟基氧化铁修正后的前 10 天内,并且稳定效果通常随着羟基氧化铁剂量的增加而增加。这可能是由于快速的 Fe-OC 吸附保护了 OC 免受微生物分解。水铁矿 ( cf. 针铁矿)对抑制由葡萄糖诱导的 SOC 矿化的正引发作用较小,这可能是由于水铁矿溶解速率较高导致 C 使用效率较低和 Fe-OC 缔合不稳定所致。添加葡萄糖后 SIC 损失增加,而 Fe 羟基氧化物进一步增强了这种损失。我们得出结论,Fe 羟基氧化物可能是增加高碱性钙溶胶中 SOC 的有用修正剂。



























 京公网安备 11010802027423号
京公网安备 11010802027423号