当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Direct Asymmetric Addition of Heteroatom Nucleophiles to Imines
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2022-05-13 , DOI: 10.1002/adsc.202200155 Ilya Egorov 1 , Sougata Santra 1 , Grigory Zyryanov 1 , Adinath Majee 2 , Alakananda Hajra 2 , Oleg Chupakhin 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2022-05-13 , DOI: 10.1002/adsc.202200155 Ilya Egorov 1 , Sougata Santra 1 , Grigory Zyryanov 1 , Adinath Majee 2 , Alakananda Hajra 2 , Oleg Chupakhin 1
Affiliation
|
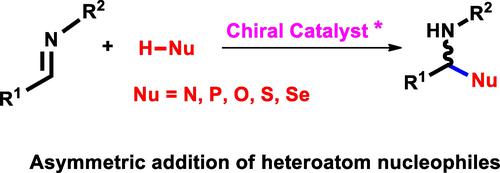
The direct asymmetric addition of heteroatom nucleophiles to imines represents one of the most efficient tools for the formation of carbon-heteroatom bonds and has received much interest recently. The review is devoted to the asymmetric addition reactions of non-carbon nucleophiles to imines. The present review is divided into five main sections: reactions with N-nucleophiles; reactions with P-nucleophiles; reactions with O-nucleophiles; reactions with S-nucleophiles; reactions with Se-nucleophiles. It has been seen that chiral catalysts such as quinines, BINOLs, chiral phosphoric acids as well as various Brønsted and Lewis acids are the source of asymmetry of the presented reactions of asymmetric addition of non-carbon nucleophiles to imines.
中文翻译:

杂原子亲核试剂与亚胺的直接不对称加成
杂原子亲核试剂与亚胺的直接不对称加成代表了形成碳-杂原子键的最有效工具之一,并且最近受到了广泛关注。该综述致力于非碳亲核试剂与亚胺的不对称加成反应。本综述分为五个主要部分:与 N-亲核试剂的反应;与 P-亲核试剂的反应;与 O-亲核试剂的反应;与 S-亲核试剂的反应;与硒亲核试剂的反应。已经看到,手性催化剂如奎宁、BINOL、手性磷酸以及各种布朗斯台德和路易斯酸是非碳亲核试剂不对称加成亚胺反应的不对称来源。
更新日期:2022-05-13
中文翻译:

杂原子亲核试剂与亚胺的直接不对称加成
杂原子亲核试剂与亚胺的直接不对称加成代表了形成碳-杂原子键的最有效工具之一,并且最近受到了广泛关注。该综述致力于非碳亲核试剂与亚胺的不对称加成反应。本综述分为五个主要部分:与 N-亲核试剂的反应;与 P-亲核试剂的反应;与 O-亲核试剂的反应;与 S-亲核试剂的反应;与硒亲核试剂的反应。已经看到,手性催化剂如奎宁、BINOL、手性磷酸以及各种布朗斯台德和路易斯酸是非碳亲核试剂不对称加成亚胺反应的不对称来源。











































 京公网安备 11010802027423号
京公网安备 11010802027423号