当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photo-Catalyzed Redox-Neutral 1,2-Dialkylation of Alkenes
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2022-05-13 , DOI: 10.1002/adsc.202200310 Wen-Hui Sun 1 , Jian-Yu Zou 1 , xiao-jing xu 2 , Jin-Lin Wang 1 , mei-ling liu 2 , Xue-yuan Liu 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2022-05-13 , DOI: 10.1002/adsc.202200310 Wen-Hui Sun 1 , Jian-Yu Zou 1 , xiao-jing xu 2 , Jin-Lin Wang 1 , mei-ling liu 2 , Xue-yuan Liu 1
Affiliation
|
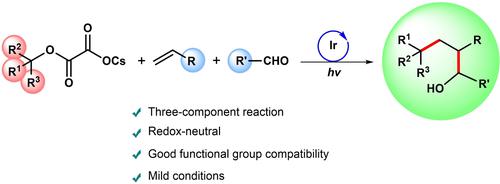
Difunctionalization of alkenes enables construction of complex compounds in one pot. In this report, we presented an intermolecular, redox-neutral three-component dicarbofunctionalization of activated alkenes under mild reaction conditions. The mechanistic studies indicated tertiary alkyl radicals from oxalate salts were first added to alkenes in Giese-type fashion, followed by nucleophilic addition with aldehydes to produce the desired 1,2-dialkylation products. The catalyst system does not require any additives, with good functional group compatibility, regioselectivity, and constructs two tertiary carbon centers simultaneously.
中文翻译:

烯烃的光催化氧化还原中性 1,2-二烷基化
烯烃的双官能化能够在一锅中构建复杂的化合物。在本报告中,我们提出了在温和反应条件下活化烯烃的分子间、氧化还原中性三组分二碳官能化。机理研究表明,草酸盐的叔烷基自由基首先以 Giese 型方式添加到烯烃中,然后与醛进行亲核加成以产生所需的 1,2-二烷基化产物。该催化剂体系不需要任何添加剂,具有良好的官能团相容性和区域选择性,同时构建了两个叔碳中心。
更新日期:2022-05-13
中文翻译:

烯烃的光催化氧化还原中性 1,2-二烷基化
烯烃的双官能化能够在一锅中构建复杂的化合物。在本报告中,我们提出了在温和反应条件下活化烯烃的分子间、氧化还原中性三组分二碳官能化。机理研究表明,草酸盐的叔烷基自由基首先以 Giese 型方式添加到烯烃中,然后与醛进行亲核加成以产生所需的 1,2-二烷基化产物。该催化剂体系不需要任何添加剂,具有良好的官能团相容性和区域选择性,同时构建了两个叔碳中心。











































 京公网安备 11010802027423号
京公网安备 11010802027423号