当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A universal strategy for high-voltage aqueous batteries via lone pair electrons as the hydrogen bond-breaker
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2022-05-13 , DOI: 10.1039/d2ee00417h Yanxin Shang 1 , Shi Chen 1 , Nan Chen 1 , Yuejiao Li 1 , Jingning Lai 1 , Yue Ma 1 , Jun Chen 1 , Feng Wu 1, 2, 3 , Renjie Chen 1, 2, 3
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2022-05-13 , DOI: 10.1039/d2ee00417h Yanxin Shang 1 , Shi Chen 1 , Nan Chen 1 , Yuejiao Li 1 , Jingning Lai 1 , Yue Ma 1 , Jun Chen 1 , Feng Wu 1, 2, 3 , Renjie Chen 1, 2, 3
Affiliation
|
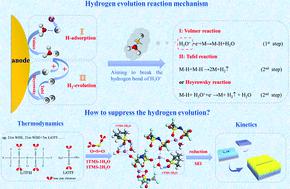
Aqueous batteries have attracted extensive attention for their safety, low cost, and non-toxicity properties. However, the narrow electrochemical stability window and freezing of water at a low temperature limit the energy density and working temperature range of aqueous Li-ion batteries. Herein, we introduce a “hydrogen bond-captured” solvent, which has lone pair electrons on the oxygen atom, to break the original water hydrogen bond network by forming intermolecular hydrogen bonds, resulting in the suppressed hydrogen evolution reaction (HER) and reduced water activity. The “LiTFSI(TMS)0.5Water” electrolyte enables stable interfacial chemistry, broadens the electrochemical stability window to 5.4 V and exhibits a freezing point lower than −85 °C. An aqueous LiNi0.5Mn1.5O4/Li4Ti5O12 full cell achieved an energy density of 136 W h kg−1 for 300 cycles at 6C. An understanding of how to alter the thermodynamics pathway of the HER in the electrolyte provides a practical guideline for designing high-voltage and wide temperature range aqueous electrolytes for sustainable energy storage applications.
中文翻译:

以孤对电子作为氢键破坏剂的高压水系电池通用策略
水系电池因其安全、低成本、无毒等特性而受到广泛关注。然而,狭窄的电化学稳定性窗口和低温下水的冻结限制了水系锂离子电池的能量密度和工作温度范围。在此,我们引入了一种“氢键捕获”溶剂,它在氧原子上具有孤对电子,通过形成分子间氢键来破坏原有的水氢键网络,从而抑制析氢反应(HER)并减少水活动。“LiTFSI(TMS) 0.5 Water”电解质可实现稳定的界面化学,将电化学稳定性窗口拓宽至 5.4 V,且冰点低于 -85 °C。水性 LiNi 0.5 Mn 1.5O 4 /Li 4 Ti 5 O 12全电池在6℃下300次循环达到136 W h kg -1的能量密度。了解如何改变电解质中 HER 的热力学途径为设计用于可持续储能应用的高压和宽温度范围水性电解质提供了实用指南。
更新日期:2022-05-13
中文翻译:

以孤对电子作为氢键破坏剂的高压水系电池通用策略
水系电池因其安全、低成本、无毒等特性而受到广泛关注。然而,狭窄的电化学稳定性窗口和低温下水的冻结限制了水系锂离子电池的能量密度和工作温度范围。在此,我们引入了一种“氢键捕获”溶剂,它在氧原子上具有孤对电子,通过形成分子间氢键来破坏原有的水氢键网络,从而抑制析氢反应(HER)并减少水活动。“LiTFSI(TMS) 0.5 Water”电解质可实现稳定的界面化学,将电化学稳定性窗口拓宽至 5.4 V,且冰点低于 -85 °C。水性 LiNi 0.5 Mn 1.5O 4 /Li 4 Ti 5 O 12全电池在6℃下300次循环达到136 W h kg -1的能量密度。了解如何改变电解质中 HER 的热力学途径为设计用于可持续储能应用的高压和宽温度范围水性电解质提供了实用指南。











































 京公网安备 11010802027423号
京公网安备 11010802027423号