当前位置:
X-MOL 学术
›
Chem. Soc. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Noncovalent interactions in proteins and nucleic acids: beyond hydrogen bonding and π-stacking
Chemical Society Reviews ( IF 40.4 ) Pub Date : 2022-05-13 , DOI: 10.1039/d2cs00133k Subhrakant Jena 1, 2 , Juhi Dutta 1, 2 , Kiran Devi Tulsiyan 1, 2 , Akshay Kumar Sahu 1, 2 , Shubhranshu Shekhar Choudhury 1, 2 , Himansu S Biswal 1, 2
Chemical Society Reviews ( IF 40.4 ) Pub Date : 2022-05-13 , DOI: 10.1039/d2cs00133k Subhrakant Jena 1, 2 , Juhi Dutta 1, 2 , Kiran Devi Tulsiyan 1, 2 , Akshay Kumar Sahu 1, 2 , Shubhranshu Shekhar Choudhury 1, 2 , Himansu S Biswal 1, 2
Affiliation
|
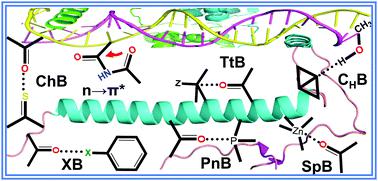
Understanding the noncovalent interactions (NCIs) among the residues of proteins and nucleic acids, and between drugs and proteins/nucleic acids, etc., has extraordinary relevance in biomolecular structure and function. It helps in interpreting the dynamics of complex biological systems and enzymatic activity, which is esential for new drug design and efficient drug delivery. NCIs like hydrogen bonding (H-bonding) and π-stacking have been researchers’ delight for a long time. Prominent among the recently discovered NCIs are halogen, chalcogen, pnictogen, tetrel, carbo-hydrogen, and spodium bonding, and n → π* interaction. These NCIs have caught the imaginations of various research groups in recent years while explaining several chemical and biological processes. At this stage, a holistic view of these new ideas and findings lying scattered can undoubtedly trigger our minds to explore more. The present review attempts to address NCIs beyond H-bonding and π-stacking, which are mainly n → σ*, n → π* and σ → σ* type interactions. Five of the seven NCIs mentioned earlier are linked to five non-inert end groups of the modern periodic table. Halogen (group-17) bonding is one of the oldest and most explored NCIs, which finds its relevance in biomolecules due to the phase correction and inhibitory properties of halogens. Chalcogen (group 16) bonding serves as a redox-active functional group of different active sites of enzymes and acts as a nucleophile in proteases and phosphates. Pnictogen (group 15), tetrel (group 14), triel (group 13) and spodium (group 12) bonding does exist in biomolecules. The n → π* interactions are linked to backbone carbonyl groups and protein side chains. Thus, they are crucial in determining the conformational stability of the secondary structures in proteins. In addition, a more recently discovered to and fro σ → σ* type interaction, namely carbo-hydrogen bonding, is also present in protein–ligand systems. This review summarizes these grand epiphanies routinely used to elucidate the structure and dynamics of biomolecules, their enzymatic activities, and their application in drug discovery. It also briefs about the future perspectives and challenges posed to the spectroscopists and theoreticians.
中文翻译:

蛋白质和核酸中的非共价相互作用:超越氢键和π-堆积
了解蛋白质与核酸残基之间、药物与蛋白质/核酸等之间的非共价相互作用(NCI)。,在生物分子结构和功能方面具有非凡的相关性。它有助于解释复杂生物系统和酶活性的动力学,这对于新药设计和有效药物递送至关重要。氢键(H-bonding)和 π-stacking 等 NCI 长期以来一直是研究人员的最爱。最近发现的 NCI 中突出的是卤素、硫属元素、pnictogen、tetrel、碳氢和钠键,以及 n → π* 相互作用。近年来,这些 NCI 在解释几种化学和生物过程的同时引起了各个研究小组的想象。在这个阶段,对这些零散的新想法和发现进行整体观察,无疑可以激发我们的思想去探索更多。本综述试图解决 H 键和 π 堆叠之外的 NCI,主要是 n → σ*,n → π* 和 σ → σ* 类型的相互作用。前面提到的七个 NCI 中有五个与现代元素周期表的五个非惰性端基有关。卤素(第 17 组)键合是最古老和探索最多的 NCI 之一,由于卤素的相位校正和抑制特性,它在生物分子中具有相关性。硫属元素(第 16 组)键合充当酶的不同活性位点的氧化还原活性官能团,并充当蛋白酶和磷酸盐中的亲核试剂。Pnictogen(第 15 组)、tetrel(第 14 组)、triel(第 13 组)和钠(第 12 组)键合确实存在于生物分子中。n → π* 相互作用与骨架羰基和蛋白质侧链相连。因此,它们对于确定蛋白质二级结构的构象稳定性至关重要。此外,最近发现的 σ → σ* 型相互作用,即碳氢键,也存在于蛋白质-配体系统中。本综述总结了这些常规用于阐明生物分子的结构和动力学、它们的酶活性以及它们在药物发现中的应用的重大顿悟。它还简要介绍了光谱学家和理论家对未来的看法和挑战。
更新日期:2022-05-13
中文翻译:

蛋白质和核酸中的非共价相互作用:超越氢键和π-堆积
了解蛋白质与核酸残基之间、药物与蛋白质/核酸等之间的非共价相互作用(NCI)。,在生物分子结构和功能方面具有非凡的相关性。它有助于解释复杂生物系统和酶活性的动力学,这对于新药设计和有效药物递送至关重要。氢键(H-bonding)和 π-stacking 等 NCI 长期以来一直是研究人员的最爱。最近发现的 NCI 中突出的是卤素、硫属元素、pnictogen、tetrel、碳氢和钠键,以及 n → π* 相互作用。近年来,这些 NCI 在解释几种化学和生物过程的同时引起了各个研究小组的想象。在这个阶段,对这些零散的新想法和发现进行整体观察,无疑可以激发我们的思想去探索更多。本综述试图解决 H 键和 π 堆叠之外的 NCI,主要是 n → σ*,n → π* 和 σ → σ* 类型的相互作用。前面提到的七个 NCI 中有五个与现代元素周期表的五个非惰性端基有关。卤素(第 17 组)键合是最古老和探索最多的 NCI 之一,由于卤素的相位校正和抑制特性,它在生物分子中具有相关性。硫属元素(第 16 组)键合充当酶的不同活性位点的氧化还原活性官能团,并充当蛋白酶和磷酸盐中的亲核试剂。Pnictogen(第 15 组)、tetrel(第 14 组)、triel(第 13 组)和钠(第 12 组)键合确实存在于生物分子中。n → π* 相互作用与骨架羰基和蛋白质侧链相连。因此,它们对于确定蛋白质二级结构的构象稳定性至关重要。此外,最近发现的 σ → σ* 型相互作用,即碳氢键,也存在于蛋白质-配体系统中。本综述总结了这些常规用于阐明生物分子的结构和动力学、它们的酶活性以及它们在药物发现中的应用的重大顿悟。它还简要介绍了光谱学家和理论家对未来的看法和挑战。











































 京公网安备 11010802027423号
京公网安备 11010802027423号