Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy ( IF 4.3 ) Pub Date : 2022-05-12 , DOI: 10.1016/j.saa.2022.121371 Guohua Yao 1 , Qing Huang 2
|
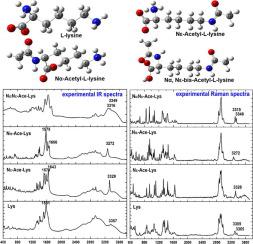
Acetylation is a common and extremely important protein modification in biology, referring to the covalent attachment of an acetyl group to the amino group. There are two forms of protein acetylation, which are lysine Nε-acetylation and N-terminal Nα-acetylation, respectively. Protein lysine Nε-acetylation is a globally important post-translational modification which plays a critical regulatory role in almost all aspects of cell metabolism. In addition, whether lysine on the N-terminal of protein can undergo Nα-acetylation is still a controversial viewpoint. Carrying out further molecular study of the role of acetylation is also the one of challenges. In order to investigate the protein acetylation more effectively, it is thus necessary to have a thorough and comprehensive understanding of lysine acetylation. In this work, both Raman and infrared (IR) spectra of L-lysine Nε-Ace-Lys, Nα-Ace-Lys, and NαNε-Ace-Lys were explored through both experimental experiment and theoretical computation based on density function theory (DFT). Vibration assignments and geometry structures of three acetylated lysines were therefore obtained for the first time in this work. The IR or Raman spectra of four molecules are very different from each other, which can be easily distinguished from the characteristic bands at 1500–1700 cm−1 and 3200–3400 cm−1 regions. Therefore, this work may provide the guide for probing the protein acetylation by Raman and IR spectroscopy.
中文翻译:

L-赖氨酸乙酰化的红外光谱和拉曼光谱的理论与实验研究
乙酰化是生物学中常见且极其重要的蛋白质修饰,指的是乙酰基与氨基的共价连接。蛋白质乙酰化有两种形式,分别是赖氨酸Nε-乙酰化和N-末端Nα-乙酰化。蛋白质赖氨酸Nε-乙酰化是一种全球重要的翻译后修饰,在细胞代谢的几乎所有方面都起着关键的调节作用。此外,蛋白质N末端的赖氨酸能否发生Nα-乙酰化仍存在争议。对乙酰化作用进行进一步的分子研究也是挑战之一。为了更有效地研究蛋白质乙酰化,因此有必要对赖氨酸乙酰化有一个彻底和全面的了解。在这项工作中,通过实验实验和基于密度函数理论(DFT)的理论计算,探索了L-赖氨酸Nε-Ace-Lys、Nα-Ace-Lys和NαNε-Ace-Lys的拉曼和红外(IR)光谱。因此,在这项工作中首次获得了三种乙酰化赖氨酸的振动分配和几何结构。四种分子的 IR 或拉曼光谱差异很大,可以很容易地从 1500-1700 cm 处的特征谱带中区分出来-1和 3200–3400 cm -1区域。因此,该工作可为利用拉曼光谱和红外光谱探测蛋白质乙酰化提供指导。











































 京公网安备 11010802027423号
京公网安备 11010802027423号