Cell Host & Microbe ( IF 20.6 ) Pub Date : 2022-05-11 , DOI: 10.1016/j.chom.2022.04.005 Jumana Samara 1 , Shirin Moossavi 2 , Belal Alshaikh 3 , Van A Ortega 4 , Veronika Kuchařová Pettersen 5 , Tahsin Ferdous 4 , Suzie L Hoops 6 , Amuchou Soraisham 3 , Joseph Vayalumkal 7 , Deonne Dersch-Mills 3 , Jeffrey S Gerber 8 , Sagori Mukhopadhyay 9 , Karen Puopolo 9 , Thomas A Tompkins 10 , Dan Knights 6 , Jens Walter 11 , Harish Amin 3 , Marie-Claire Arrieta 4
|
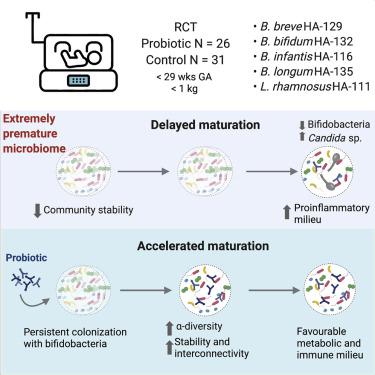
Probiotics are increasingly administered to premature infants to prevent necrotizing enterocolitis and neonatal sepsis. However, their effects on gut microbiome assembly and immunity are poorly understood. Using a randomized intervention trial in extremely premature infants, we tested the effects of a probiotic product containing four strains of Bifidobacterium species autochthonous to the infant gut and one Lacticaseibacillus strain on the compositional and functional trajectory of microbiome. Daily administration of the mixture accelerated the transition into a mature, term-like microbiome with higher stability and species interconnectivity. Besides infant age, Bifidobacterium strains and stool metabolites were the best predictors of microbiome maturation, and structural equation modeling confirmed probiotics as a major determinant for the trajectory of microbiome assembly. Bifidobacterium-driven microbiome maturation was also linked to an anti-inflammatory intestinal immune milieu. This demonstrates that Bifidobacterium strains are ecosystem engineers that lead to an acceleration of microbiome maturation and immunological consequences in extremely premature infants.
中文翻译:

补充益生菌混合物可加速肠道微生物群的成熟并减少极早产儿的肠道炎症
益生菌越来越多地用于早产儿以预防坏死性小肠结肠炎和新生儿败血症。然而,人们对它们对肠道微生物组组装和免疫的影响知之甚少。通过一项针对极早产儿的随机干预试验,我们测试了一种益生菌产品对婴儿肠道的四种原产双歧杆菌菌株和一种乳酸杆菌菌株的益生菌产品对微生物组的组成和功能轨迹的影响。每天服用混合物加速了向成熟的、类似术语的微生物组的转变,具有更高的稳定性和物种相互联系。除了婴儿年龄,双歧杆菌菌株和粪便代谢物是微生物组成熟的最佳预测因子,结构方程模型证实益生菌是微生物组组装轨迹的主要决定因素。双歧杆菌驱动的微生物组成熟也与抗炎肠道免疫环境有关。这表明双歧杆菌菌株是生态系统工程师,可加速极早产儿的微生物组成熟和免疫后果。










































 京公网安备 11010802027423号
京公网安备 11010802027423号