当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Designing supercapacitor electrolyte via ion counting
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2022-05-11 , DOI: 10.1039/d2ee00376g Shrisudersan Jayaraman 1 , Travis J. Rawson 2 , Marina A. Belyustina 1
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2022-05-11 , DOI: 10.1039/d2ee00376g Shrisudersan Jayaraman 1 , Travis J. Rawson 2 , Marina A. Belyustina 1
Affiliation
|
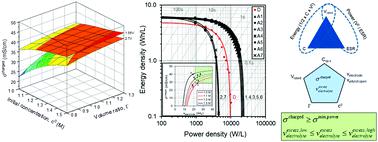
Supercapacitors or electrochemical double layer capacitors (EDLCs) are energy storage devices with moderate energy and high power densities and can act complementarily to batteries which have high energy and low power densities, in applications like electric vehicles. Significant effort is spent on developing and improving supercapacitor materials like activated carbon, electrolytes, and separators. However, high-performing materials do not always translate to high performing devices. This work describes a theoretical framework coupled with experiments that establishes two necessary and sufficient conditions to build supercapacitors with optimized performance. Establishing lower bounds for the electrolyte conductivity when the device is at a fully charged state and the excess electrolyte volume automatically apply constraints on the material, process, and design variables. For a Type A electrode system (specific capacitance = 75 F cm−3), the lower bounds for conductivity and excess electrolyte volume were found to be 28 mS cm−1 and 3 mL for a D-cell sized supercapacitor. The underlying philosophy of counting ions and matching it with the number of electrons to optimize materials can be modified for individual systems (for example, by introducing Faradaic reaction components for pseudocapacitors) and be broadly applied to energy storage technologies such as pseudocapacitors, lithium batteries and redox flow batteries.
中文翻译:

通过离子计数设计超级电容器电解质
超级电容器或电化学双层电容器 (EDLC) 是具有中等能量和高功率密度的储能装置,可以在电动汽车等应用中与具有高能量和低功率密度的电池互补。在开发和改进活性炭、电解质和隔膜等超级电容器材料方面投入了大量精力。然而,高性能材料并不总能转化为高性能设备。这项工作描述了一个理论框架和实验,该框架建立了两个必要和充分的条件来构建具有优化性能的超级电容器。当设备处于完全充电状态并且过量的电解质体积自动对材料施加约束时,建立电解质电导率的下限,过程和设计变量。对于 A 型电极系统(比电容 = 75 F cm-3 ),对于 D 电池大小的超级电容器,发现电导率和过量电解质体积的下限为 28 mS cm -1和 3 mL。计算离子并将其与电子数匹配以优化材料的基本原理可以针对单个系统进行修改(例如,通过为赝电容器引入法拉第反应组件),并广泛应用于赝电容器、锂电池和锂电池等储能技术。氧化还原液流电池。
更新日期:2022-05-11
中文翻译:

通过离子计数设计超级电容器电解质
超级电容器或电化学双层电容器 (EDLC) 是具有中等能量和高功率密度的储能装置,可以在电动汽车等应用中与具有高能量和低功率密度的电池互补。在开发和改进活性炭、电解质和隔膜等超级电容器材料方面投入了大量精力。然而,高性能材料并不总能转化为高性能设备。这项工作描述了一个理论框架和实验,该框架建立了两个必要和充分的条件来构建具有优化性能的超级电容器。当设备处于完全充电状态并且过量的电解质体积自动对材料施加约束时,建立电解质电导率的下限,过程和设计变量。对于 A 型电极系统(比电容 = 75 F cm-3 ),对于 D 电池大小的超级电容器,发现电导率和过量电解质体积的下限为 28 mS cm -1和 3 mL。计算离子并将其与电子数匹配以优化材料的基本原理可以针对单个系统进行修改(例如,通过为赝电容器引入法拉第反应组件),并广泛应用于赝电容器、锂电池和锂电池等储能技术。氧化还原液流电池。











































 京公网安备 11010802027423号
京公网安备 11010802027423号