当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Highly Enantioselective Hydroxylation of 3-Arylpropanenitriles to Access Chiral β-Hydroxy Nitriles by Engineering of P450pyr Monooxygenase
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2022-05-10 , DOI: 10.1021/acs.oprd.1c00444 Guo-Zhong Deng 1, 2 , Xu Zhou 1, 2 , Quan-Xiang Yu 1, 2 , Xue-Qing Mou 1, 2 , Miao An 1, 2 , Hai-Bo Cui 1, 2 , Xiao-Jian Zhou 1, 2 , Nan-Wei Wan 1, 2 , Zhi Li 3 , Yong-Zheng Chen 1, 2
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2022-05-10 , DOI: 10.1021/acs.oprd.1c00444 Guo-Zhong Deng 1, 2 , Xu Zhou 1, 2 , Quan-Xiang Yu 1, 2 , Xue-Qing Mou 1, 2 , Miao An 1, 2 , Hai-Bo Cui 1, 2 , Xiao-Jian Zhou 1, 2 , Nan-Wei Wan 1, 2 , Zhi Li 3 , Yong-Zheng Chen 1, 2
Affiliation
|
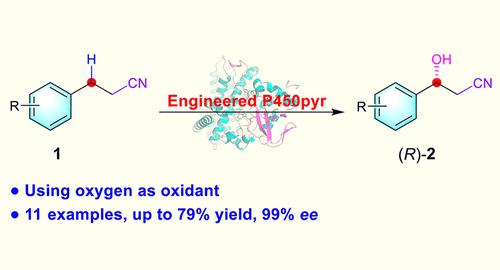
Direct enzymatic hydroxylation of prochiral alkylnitriles represents a green and efficient synthesis of chiral β-hydroxy nitriles, but reported examples are very inefficient and limited. Here we describe the development of an evolved P450pyr monooxygenase for enantioselective benzylic hydroxylation of alkylnitriles. By the use of an engineered P450pyr-D5M3 mutant, a variety of pharmaceutically relevant chiral aryl-substituted β-hydroxy nitriles were prepared from prochiral 3-arylpropanenitriles in moderate yields with excellent enantioselectivities.
中文翻译:

通过 P450pyr 单加氧酶工程对 3-芳基丙腈进行高对映选择性羟基化制备手性 β-羟基腈
前手性烷基腈的直接酶促羟基化代表了手性 β-羟基腈的绿色高效合成,但报道的例子非常低效且有限。在这里,我们描述了一种进化的 P450pyr 单加氧酶,用于烷基腈的对映选择性苄基羟基化。通过使用工程化的 P450pyr-D5M3 突变体,从前手性 3-芳基丙腈以中等收率和优异的对映选择性制备了多种药学相关的手性芳基取代的 β-羟基腈。
更新日期:2022-05-10
中文翻译:

通过 P450pyr 单加氧酶工程对 3-芳基丙腈进行高对映选择性羟基化制备手性 β-羟基腈
前手性烷基腈的直接酶促羟基化代表了手性 β-羟基腈的绿色高效合成,但报道的例子非常低效且有限。在这里,我们描述了一种进化的 P450pyr 单加氧酶,用于烷基腈的对映选择性苄基羟基化。通过使用工程化的 P450pyr-D5M3 突变体,从前手性 3-芳基丙腈以中等收率和优异的对映选择性制备了多种药学相关的手性芳基取代的 β-羟基腈。











































 京公网安备 11010802027423号
京公网安备 11010802027423号