当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Fit-for-Purpose Synthesis of an Aza-Cryptophycin Analogue as the Payload for an Antibody–Drug Conjugate
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2022-05-10 , DOI: 10.1021/acs.oprd.2c00080 Audrey Bendelac 1 , Françoise Benedetti 2 , Valerie Doublet-Decabras 1 , Rachel Lokovi 2 , François Decalogne 2 , Antony Bigot 2
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2022-05-10 , DOI: 10.1021/acs.oprd.2c00080 Audrey Bendelac 1 , Françoise Benedetti 2 , Valerie Doublet-Decabras 1 , Rachel Lokovi 2 , François Decalogne 2 , Antony Bigot 2
Affiliation
|
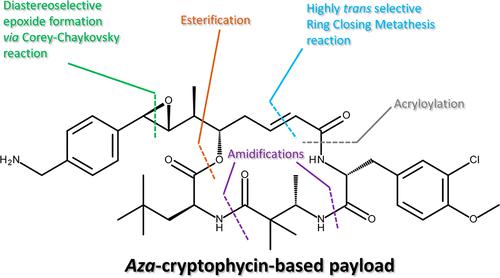
The synthesis of an aza-cryptophycin analogue is described, featuring a highly trans-selective ring-closing metathesis reaction and an asymmetric Corey–Chaykovsky-type reaction to install the epoxide function. This latter reaction is an answer to the long-standing synthetic challenged posed by the efficient formation of the epoxide function of the cryptophycin family of compounds in a diastereoselective manner. It allows for a one-third reduction in the number of steps compared with the previously used synthesis (27 to 19 steps) and increases the overall yield of the longest linear sequence by a factor of 27 (0.6% to 16%). This fit-for-purpose synthesis allowed the production of a steady supply of the material for the early phase of a cryptophycin-based antibody–drug conjugate program.
中文翻译:

作为抗体-药物偶联物有效载荷的氮杂-隐藻素类似物的专用合成
描述了氮杂隐藻素类似物的合成,具有高度反式选择性闭环复分解反应和不对称 Corey-Chaykovsky 型反应以安装环氧化物功能。后一种反应是对由以非对映选择性方式有效形成隐藻素家族化合物的环氧化物功能所带来的长期合成挑战的答案。与之前使用的合成(27 到 19 步)相比,它允许步骤数减少三分之一,并将最长线性序列的总产率提高 27 倍(0.6% 到 16%)。这种适合目的的合成允许为基于隐藻素的抗体-药物偶联计划的早期阶段稳定供应材料。
更新日期:2022-05-10
中文翻译:

作为抗体-药物偶联物有效载荷的氮杂-隐藻素类似物的专用合成
描述了氮杂隐藻素类似物的合成,具有高度反式选择性闭环复分解反应和不对称 Corey-Chaykovsky 型反应以安装环氧化物功能。后一种反应是对由以非对映选择性方式有效形成隐藻素家族化合物的环氧化物功能所带来的长期合成挑战的答案。与之前使用的合成(27 到 19 步)相比,它允许步骤数减少三分之一,并将最长线性序列的总产率提高 27 倍(0.6% 到 16%)。这种适合目的的合成允许为基于隐藻素的抗体-药物偶联计划的早期阶段稳定供应材料。











































 京公网安备 11010802027423号
京公网安备 11010802027423号