当前位置:
X-MOL 学术
›
Chem. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
1H-Detected Biomolecular NMR under Fast Magic-Angle Spinning
Chemical Reviews ( IF 51.4 ) Pub Date : 2022-05-10 , DOI: 10.1021/acs.chemrev.1c00918 Tanguy Le Marchand 1 , Tobias Schubeis 1 , Marta Bonaccorsi 1, 2 , Piotr Paluch 3 , Daniela Lalli 4 , Andrew J Pell 1, 5 , Loren B Andreas 6 , Kristaps Jaudzems 7, 8 , Jan Stanek 3 , Guido Pintacuda 1
Chemical Reviews ( IF 51.4 ) Pub Date : 2022-05-10 , DOI: 10.1021/acs.chemrev.1c00918 Tanguy Le Marchand 1 , Tobias Schubeis 1 , Marta Bonaccorsi 1, 2 , Piotr Paluch 3 , Daniela Lalli 4 , Andrew J Pell 1, 5 , Loren B Andreas 6 , Kristaps Jaudzems 7, 8 , Jan Stanek 3 , Guido Pintacuda 1
Affiliation
|
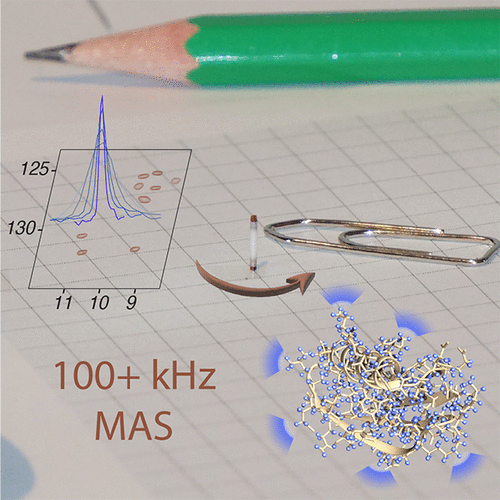
Since the first pioneering studies on small deuterated peptides dating more than 20 years ago, 1H detection has evolved into the most efficient approach for investigation of biomolecular structure, dynamics, and interactions by solid-state NMR. The development of faster and faster magic-angle spinning (MAS) rates (up to 150 kHz today) at ultrahigh magnetic fields has triggered a real revolution in the field. This new spinning regime reduces the 1H–1H dipolar couplings, so that a direct detection of 1H signals, for long impossible without proton dilution, has become possible at high resolution. The switch from the traditional MAS NMR approaches with 13C and 15N detection to 1H boosts the signal by more than an order of magnitude, accelerating the site-specific analysis and opening the way to more complex immobilized biological systems of higher molecular weight and available in limited amounts. This paper reviews the concepts underlying this recent leap forward in sensitivity and resolution, presents a detailed description of the experimental aspects of acquisition of multidimensional correlation spectra with fast MAS, and summarizes the most successful strategies for the assignment of the resonances and for the elucidation of protein structure and conformational dynamics. It finally outlines the many examples where 1H-detected MAS NMR has contributed to the detailed characterization of a variety of crystalline and noncrystalline biomolecular targets involved in biological processes ranging from catalysis through drug binding, viral infectivity, amyloid fibril formation, to transport across lipid membranes.
中文翻译:

快速魔角旋转下的 1H 检测生物分子 NMR
自 20 多年前首次对小氘化肽进行开创性研究以来,1 H 检测已发展成为通过固态 NMR 研究生物分子结构、动力学和相互作用的最有效方法。在超高磁场下越来越快的魔角旋转 (MAS) 速率(目前高达 150 kHz)的发展引发了该领域的真正革命。这种新的旋转机制减少了1 H– 1 H 偶极耦合,因此长期以来在没有质子稀释的情况下不可能直接检测1 H 信号,现在已经可以以高分辨率进行。从传统的13 C 和15 N 检测到1 H的 MAS NMR 方法的转变将信号增强了一个数量级以上,加速了位点特异性分析,并为更复杂的更高分子量和更高分子量的固定化生物系统开辟了道路。数量有限。本文回顾了最近灵敏度和分辨率方面飞跃的概念,详细描述了使用快速 MAS 采集多维相关光谱的实验方面,并总结了共振分配和阐明共振的最成功策略。蛋白质结构和构象动力学。最后概述了许多示例,其中1 H 检测的 MAS NMR 有助于详细表征生物过程中涉及的各种结晶和非结晶生物分子靶标,这些生物过程包括从催化到药物结合、病毒感染性、淀粉样蛋白纤维形成,再到跨脂质运输膜。
更新日期:2022-05-10
中文翻译:

快速魔角旋转下的 1H 检测生物分子 NMR
自 20 多年前首次对小氘化肽进行开创性研究以来,1 H 检测已发展成为通过固态 NMR 研究生物分子结构、动力学和相互作用的最有效方法。在超高磁场下越来越快的魔角旋转 (MAS) 速率(目前高达 150 kHz)的发展引发了该领域的真正革命。这种新的旋转机制减少了1 H– 1 H 偶极耦合,因此长期以来在没有质子稀释的情况下不可能直接检测1 H 信号,现在已经可以以高分辨率进行。从传统的13 C 和15 N 检测到1 H的 MAS NMR 方法的转变将信号增强了一个数量级以上,加速了位点特异性分析,并为更复杂的更高分子量和更高分子量的固定化生物系统开辟了道路。数量有限。本文回顾了最近灵敏度和分辨率方面飞跃的概念,详细描述了使用快速 MAS 采集多维相关光谱的实验方面,并总结了共振分配和阐明共振的最成功策略。蛋白质结构和构象动力学。最后概述了许多示例,其中1 H 检测的 MAS NMR 有助于详细表征生物过程中涉及的各种结晶和非结晶生物分子靶标,这些生物过程包括从催化到药物结合、病毒感染性、淀粉样蛋白纤维形成,再到跨脂质运输膜。









































 京公网安备 11010802027423号
京公网安备 11010802027423号