当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
5-(4-Pyridinyl)-3-isothiazolols as Competitive Antagonists of Insect GABA Receptors: Design, Synthesis, and a New Mechanism Leading to Insecticidal Effects
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2022-05-10 , DOI: 10.1021/acs.jafc.1c08030 Cheng Huang 1 , Yun Wu 1 , Na Zhai 1 , Xiulian Ju 1 , Chunqing Zhao 2 , Xiaogang Luo 1, 3 , Yoshihisa Ozoe 4 , Genyan Liu 1
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2022-05-10 , DOI: 10.1021/acs.jafc.1c08030 Cheng Huang 1 , Yun Wu 1 , Na Zhai 1 , Xiulian Ju 1 , Chunqing Zhao 2 , Xiaogang Luo 1, 3 , Yoshihisa Ozoe 4 , Genyan Liu 1
Affiliation
|
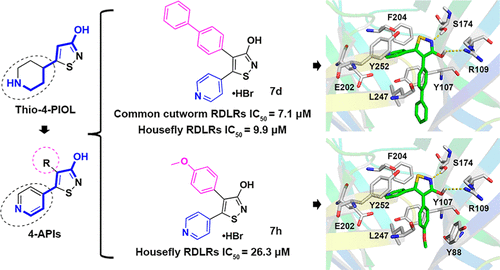
Ionotropic γ-aminobutyric acid (GABA) receptors (iGABARs) are validated targets of drugs and insecticides. Our previous studies showed that the competitive antagonists of insect iGABARs exhibit insecticidal activities and that the 3-isothiazolol scaffold is used as a lead for developing novel iGABAR antagonists. Here, we designed a novel series of 4-aryl-5-(4-pyridinyl)-3-isothiazolol (4-API) analogs that have various aromatic substituents at the 4-position. Two-electrode voltage clamp experiments showed that all synthesized 4-APIs exhibited antagonistic activity against Musca domestica and Spodoptera litura iGABARs (RDL) expressed in oocytes of Xenopus laevis at 100 μM. Of the 4-APIs, the 4-(1,1′-biphenylyl) analog was the most potent antagonist with IC50s of 7.1 and 9.9 μM against M. domestica and S. litura RDL receptors, respectively. This analog also showed a certain insecticidal activity against S. litura larvae, with >75% mortality at 100 μg/g diet. Molecular docking studies with a M. domestica iGABAR model indicated that the π–π stacking interactions formed between the pyridinyl ring and Y252 and between the 4-substituted aromatic group and Y107 might be important for antagonism by the 4-(1,1′-biphenylyl) analog. Our studies provide important information for designing novel iGABAR antagonists and suggest that the 4-APIs acting on iGABARs are promising insecticide leads for further studies.
中文翻译:

5-(4-Pyridinyl)-3-isothiazolols 作为昆虫 GABA 受体的竞争性拮抗剂:设计、合成和导致杀虫效果的新机制
离子型 γ-氨基丁酸 (GABA) 受体 (iGABAR) 是药物和杀虫剂的有效靶标。我们之前的研究表明,昆虫 iGABAR 的竞争性拮抗剂具有杀虫活性,并且 3-异噻唑醇支架被用作开发新型 iGABAR 拮抗剂的先导。在这里,我们设计了一系列新的 4-aryl-5-(4-pyridinyl)-3-isothiazolol (4-API) 类似物,它们在 4 位具有各种芳香族取代基。双电极电压钳实验表明,所有合成的 4-API在 100 μM 浓度下均对在非洲爪蟾卵母细胞中表达的家蝇和斜纹夜蛾iGABARs (RDL) 具有拮抗活性。在 4-API 中,4-(1,1'-联苯基) 类似物是最有效的 IC 拮抗剂50 s 的 7.1 和 9.9 μM 分别针对M. domestica和S. litura RDL 受体。该类似物还显示出对S. litura幼虫的一定杀虫活性,在 100 μg/g 饮食中死亡率 > 75%。用家蚕iGABAR模型进行的分子对接研究表明,吡啶环与 Y252 之间以及 4-取代芳香基团与 Y107 之间形成的 π-π 堆积相互作用可能对 4-(1,1'-联苯基)类似物。我们的研究为设计新型 iGABAR 拮抗剂提供了重要信息,并表明作用于 iGABAR 的 4-API 是有前途的杀虫剂,可用于进一步研究。
更新日期:2022-05-10
中文翻译:

5-(4-Pyridinyl)-3-isothiazolols 作为昆虫 GABA 受体的竞争性拮抗剂:设计、合成和导致杀虫效果的新机制
离子型 γ-氨基丁酸 (GABA) 受体 (iGABAR) 是药物和杀虫剂的有效靶标。我们之前的研究表明,昆虫 iGABAR 的竞争性拮抗剂具有杀虫活性,并且 3-异噻唑醇支架被用作开发新型 iGABAR 拮抗剂的先导。在这里,我们设计了一系列新的 4-aryl-5-(4-pyridinyl)-3-isothiazolol (4-API) 类似物,它们在 4 位具有各种芳香族取代基。双电极电压钳实验表明,所有合成的 4-API在 100 μM 浓度下均对在非洲爪蟾卵母细胞中表达的家蝇和斜纹夜蛾iGABARs (RDL) 具有拮抗活性。在 4-API 中,4-(1,1'-联苯基) 类似物是最有效的 IC 拮抗剂50 s 的 7.1 和 9.9 μM 分别针对M. domestica和S. litura RDL 受体。该类似物还显示出对S. litura幼虫的一定杀虫活性,在 100 μg/g 饮食中死亡率 > 75%。用家蚕iGABAR模型进行的分子对接研究表明,吡啶环与 Y252 之间以及 4-取代芳香基团与 Y107 之间形成的 π-π 堆积相互作用可能对 4-(1,1'-联苯基)类似物。我们的研究为设计新型 iGABAR 拮抗剂提供了重要信息,并表明作用于 iGABAR 的 4-API 是有前途的杀虫剂,可用于进一步研究。











































 京公网安备 11010802027423号
京公网安备 11010802027423号