当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Manufacturable Process of a Novel EGFR Inhibitor (Larotinib) for the Treatment of ESCC
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2022-05-09 , DOI: 10.1021/acs.oprd.2c00059 Weihong Zhang 1 , Zhiming Su 2 , Haiwang Liu 2 , Yingjun Zhang 2 , Weiliang Ye 2 , Dahua Peng 2 , Hongpeng Xie 2 , Hongtao Peng 2 , Zhihong Peng 1 , Wanrong Dong 1 , Delie An 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2022-05-09 , DOI: 10.1021/acs.oprd.2c00059 Weihong Zhang 1 , Zhiming Su 2 , Haiwang Liu 2 , Yingjun Zhang 2 , Weiliang Ye 2 , Dahua Peng 2 , Hongpeng Xie 2 , Hongtao Peng 2 , Zhihong Peng 1 , Wanrong Dong 1 , Delie An 1
Affiliation
|
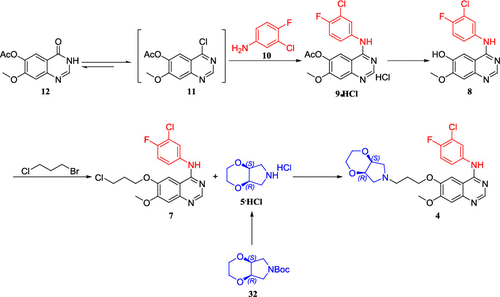
The development of an efficient synthetic process for a clinical candidate Larotinib (4), which is an epidermal growth factor receptor (EGFR) inhibitor for the treatment of esophageal squamous cell carcinoma (ESCC), is reported for scale-up. The process used 3,4-dihydro-7-methoxy-4-oxoquinazolin-6-yl acetate (12) as the regulatory starting material and provided a stable and industrializable intermediate chloroquinazoline 11 under the process control. Further optimization of the process obviously improved the reaction yield and reduced the impurity level including alkyl halide potential genotoxic impurities (PGIs), at the same time avoiding the use of laborious and time-consuming column chromatography. More than 110 kg of Larotinib (4) in one batch can be finally produced stably for clinical research. Compared to our initial synthetic route in preclinical research, the overall yield of this optimized process increased significantly from 16.2 to 55.6%.
中文翻译:

用于治疗 ESCC 的新型 EGFR 抑制剂(拉罗替尼)的可制造工艺
据报道,一种用于治疗食管鳞状细胞癌 (ESCC) 的表皮生长因子受体 (EGFR) 抑制剂临床候选药物拉罗替尼 ( 4 )的有效合成工艺的开发用于扩大规模。该工艺以3,4-二氢-7-甲氧基-4-氧代喹唑啉-6-乙酸酯( 12 )为调控起始原料,在工艺控制下提供了稳定、可工业化的中间体氯喹唑啉11 。工艺的进一步优化明显提高了反应收率并降低了包括卤代烷潜在遗传毒性杂质(PGI)在内的杂质水平,同时避免了使用费力且耗时的柱层析。超过 110 公斤的拉罗替尼 ( 4) 在一批中最终可以稳定生产用于临床研究。与我们在临床前研究中的初始合成路线相比,这一优化工艺的总产率从 16.2% 显着提高至 55.6%。
更新日期:2022-05-09
中文翻译:

用于治疗 ESCC 的新型 EGFR 抑制剂(拉罗替尼)的可制造工艺
据报道,一种用于治疗食管鳞状细胞癌 (ESCC) 的表皮生长因子受体 (EGFR) 抑制剂临床候选药物拉罗替尼 ( 4 )的有效合成工艺的开发用于扩大规模。该工艺以3,4-二氢-7-甲氧基-4-氧代喹唑啉-6-乙酸酯( 12 )为调控起始原料,在工艺控制下提供了稳定、可工业化的中间体氯喹唑啉11 。工艺的进一步优化明显提高了反应收率并降低了包括卤代烷潜在遗传毒性杂质(PGI)在内的杂质水平,同时避免了使用费力且耗时的柱层析。超过 110 公斤的拉罗替尼 ( 4) 在一批中最终可以稳定生产用于临床研究。与我们在临床前研究中的初始合成路线相比,这一优化工艺的总产率从 16.2% 显着提高至 55.6%。











































 京公网安备 11010802027423号
京公网安备 11010802027423号