当前位置:
X-MOL 学术
›
Chin. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A One-Pot Strategy for the Synthesis of β-Substituted Rhoda- and Irida-Carbolong Complexes
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2022-05-04 , DOI: 10.1002/cjoc.202200179 Binbin Xu 1 , Wei Mao 1 , Chengcheng Wu 1 , Jinhua Li 1 , Zhengyu Lu 1 , Ming Luo 1 , Dafa Chen 1 , Haiping Xia 1
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2022-05-04 , DOI: 10.1002/cjoc.202200179 Binbin Xu 1 , Wei Mao 1 , Chengcheng Wu 1 , Jinhua Li 1 , Zhengyu Lu 1 , Ming Luo 1 , Dafa Chen 1 , Haiping Xia 1
Affiliation
|
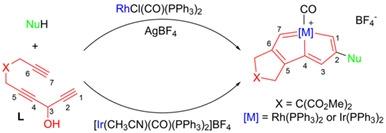
Starting from an organic multiyne, three steps are normally needed for the preparation of non-β-phosphonium functionalized rhoda- and irida-carbolong complexes. Herein, a one-pot strategy, by mixing a multiyne, a nucleophile, and RhCl(CO)(PPh3)2/AgBF4 or [Ir(CH3CN)(CO)(PPh3)2]BF4, was developed to achieve a series of β-functionalized rhoda- and irida-carbolong complexes. The β-substituents in these complexes can be various C-, N- and O-centered groups, dependent on the nucleophiles used. This strategy provides a new convenient route to construct carbolong complexes, which is important for the further development of carbolong chemistry.
中文翻译:

β-取代的 Rhoda- 和 Irida-Carbolong 配合物的一锅法合成
从有机多炔开始,制备非β-鏻官能化的铑-和铱-碳隆络合物通常需要三个步骤。在此,通过混合多炔、亲核试剂和RhCl(CO)(PPh 3 ) 2 /AgBF 4或[Ir(CH 3 CN)(CO)(PPh 3 ) 2 ]BF 4的一锅策略是开发以实现一系列β-功能化的rhoda-和irida-carbolong复合物。β _这些配合物中的取代基可以是各种以 C、N 和 O 为中心的基团,这取决于所使用的亲核试剂。该策略为构建carbolong配合物提供了一条新的便捷途径,对carbolong化学的进一步发展具有重要意义。
更新日期:2022-05-04

中文翻译:

β-取代的 Rhoda- 和 Irida-Carbolong 配合物的一锅法合成
从有机多炔开始,制备非β-鏻官能化的铑-和铱-碳隆络合物通常需要三个步骤。在此,通过混合多炔、亲核试剂和RhCl(CO)(PPh 3 ) 2 /AgBF 4或[Ir(CH 3 CN)(CO)(PPh 3 ) 2 ]BF 4的一锅策略是开发以实现一系列β-功能化的rhoda-和irida-carbolong复合物。β _这些配合物中的取代基可以是各种以 C、N 和 O 为中心的基团,这取决于所使用的亲核试剂。该策略为构建carbolong配合物提供了一条新的便捷途径,对carbolong化学的进一步发展具有重要意义。












































 京公网安备 11010802027423号
京公网安备 11010802027423号