Chem Catalysis Pub Date : 2022-05-04 , DOI: 10.1016/j.checat.2022.04.006 Weiwei Chai 1 , Bin Guo 1 , Qinglong Zhang 1 , Weiwei Zi 1, 2
|
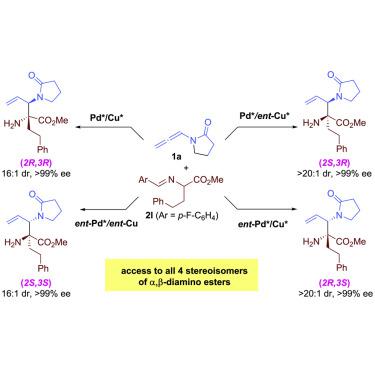
Mannich reaction is a significant transformation to prepare chiral amines from imine; however, vinylimines are still unaddressed substrates in this transformation due to the inherent competition between the Mannich and Michael addition. Herein we report a cooperative Pd/Cu-catalyzed enantioselective coupling of allenamides with aldimine esters for expeditious access to Mannich-type motifs α,β-diamino esters. The reaction has excellent regioselectivity, and by selecting the combinations of Pd and Cu catalysts with appropriate chiralities, both the syn- and anti-coupling products could be selectively prepared with high enantio-, diastereoselectivities. This work represents a rare example of employing cooperative catalysis for achieving diastereodivergent Mannich-type coupling reaction and would inspire further studies in this field.
中文翻译:

Pd/Cu 催化的丙二酰胺和醛亚胺酯的非对映发散偶联获得曼尼希型基序
曼尼希反应是由亚胺制备手性胺的重大转变;然而,由于 Mannich 和 Michael 加成之间的内在竞争,乙烯基亚胺在这种转变中仍然是未解决的底物。在这里,我们报告了一种 Pd/Cu 催化的丙二酰胺与醛亚胺酯的对映选择性偶联,以快速获得曼尼希型基序 α,β-二氨基酯。该反应具有优异的区域选择性,通过选择具有适当手性的 Pd 和 Cu 催化剂的组合,可以选择性地制备具有高对映选择性、非对映选择性的偶联产物。这项工作代表了利用协同催化实现非对映发散曼尼希型偶联反应的罕见例子,并将激发该领域的进一步研究。



























 京公网安备 11010802027423号
京公网安备 11010802027423号