Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Microfluidic harvesting of breast cancer tumor spheroid-derived extracellular vesicles from immobilized microgels for single-vesicle analysis
Lab on a Chip ( IF 6.1 ) Pub Date : 2022-05-05 , DOI: 10.1039/d1lc01053k Xilal Y Rima 1 , Jingjing Zhang 1 , Luong T H Nguyen 1 , Aaron Rajasuriyar 1 , Min Jin Yoon 1 , Chi-Ling Chiang 1 , Nicole Walters 1 , Kwang Joo Kwak 2 , L James Lee 1, 2 , Eduardo Reátegui 1, 3
Lab on a Chip ( IF 6.1 ) Pub Date : 2022-05-05 , DOI: 10.1039/d1lc01053k Xilal Y Rima 1 , Jingjing Zhang 1 , Luong T H Nguyen 1 , Aaron Rajasuriyar 1 , Min Jin Yoon 1 , Chi-Ling Chiang 1 , Nicole Walters 1 , Kwang Joo Kwak 2 , L James Lee 1, 2 , Eduardo Reátegui 1, 3
Affiliation
|
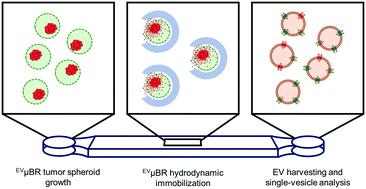
Investigating cellular and vesicular heterogeneity in breast cancer remains a challenge, which encourages the development of controllable in vitro systems that mimic the tumor microenvironment. Although three-dimensional cell culture better recapitulates the heterogeneity observed in tumor growth and extracellular vesicle (EV) biogenesis, the physiological relevance is often contrasted with the control offered by two-dimensional cell culture. Therefore, to challenge this misconception we developed a novel microfluidic system harboring highly tunable three-dimensional EV microbioreactors (EVμBRs) to model micrometastatic EV release in breast cancer while capitalizing on the convenient, low-volume, and sterile interface provided by microfluidics. The diameter and cellular occupancy of the EVμBRs could be precisely tailored to various configurations, supporting the formation of breast cancer tumor spheroids. To immobilize the EVμBRs within a microchannel and facilitate EV extraction, oxygen inhibition in free-radical polymerization was repurposed to rapidly generate two-layer hydrodynamic traps in situ using a digital-micromirror device (DMD)-based ultraviolet (UV) projection system. Breast cancer tumor spheroid-derived EVs were harvested with as little as 20 μL from the microfluidic system and quantified by single-EV immunofluorescence for CD63 and CD81. Despite the low-volume extraction, differences in biomarker expression and coexpression of the tetraspanins on single EVs were observed. Furthermore, the EVμBRs were capable of recapitulating heterogeneity at a cellular and vesicular degree, indicating the utility and robustness of the microfluidic system to investigate physiologically relevant EVs in breast cancer and other disease models.
中文翻译:

从固定化微凝胶中微流体采集乳腺癌肿瘤球体来源的细胞外囊泡用于单囊泡分析
研究乳腺癌中的细胞和囊泡异质性仍然是一个挑战,这鼓励了模拟肿瘤微环境的可控体外系统的开发。尽管三维细胞培养更好地概括了在肿瘤生长和细胞外囊泡(EV)生物发生中观察到的异质性,但其生理相关性通常与二维细胞培养提供的对照形成对比。因此,为了挑战这种误解,我们开发了一种新型微流体系统,该系统包含高度可调的三维 EV 微生物反应器 ( EV μBR),以模拟乳腺癌中微转移 EV 的释放,同时利用微流体提供的方便、小体积和无菌接口。 EV μBR 的直径和细胞占有率可以根据各种配置精确定制,支持乳腺癌肿瘤球体的形成。为了将EV μBR 固定在微通道内并促进 EV 提取,自由基聚合中的氧抑制被重新调整,使用基于数字微镜器件 (DMD) 的紫外线 (UV) 投影系统在原位快速生成两层流体动力学陷阱。从微流体系统中收获仅 20 μL 的乳腺癌肿瘤球体来源的 EV,并通过单 EV 免疫荧光对 CD63 和 CD81 进行定量。尽管提取量较低,但仍观察到单个 EV 上生物标志物表达和四跨膜蛋白共表达的差异。 此外, EV μBR 能够在细胞和囊泡层面重现异质性,表明微流体系统在研究乳腺癌和其他疾病模型中生理相关 EV 方面的实用性和鲁棒性。
更新日期:2022-05-05
中文翻译:

从固定化微凝胶中微流体采集乳腺癌肿瘤球体来源的细胞外囊泡用于单囊泡分析
研究乳腺癌中的细胞和囊泡异质性仍然是一个挑战,这鼓励了模拟肿瘤微环境的可控体外系统的开发。尽管三维细胞培养更好地概括了在肿瘤生长和细胞外囊泡(EV)生物发生中观察到的异质性,但其生理相关性通常与二维细胞培养提供的对照形成对比。因此,为了挑战这种误解,我们开发了一种新型微流体系统,该系统包含高度可调的三维 EV 微生物反应器 ( EV μBR),以模拟乳腺癌中微转移 EV 的释放,同时利用微流体提供的方便、小体积和无菌接口。 EV μBR 的直径和细胞占有率可以根据各种配置精确定制,支持乳腺癌肿瘤球体的形成。为了将EV μBR 固定在微通道内并促进 EV 提取,自由基聚合中的氧抑制被重新调整,使用基于数字微镜器件 (DMD) 的紫外线 (UV) 投影系统在原位快速生成两层流体动力学陷阱。从微流体系统中收获仅 20 μL 的乳腺癌肿瘤球体来源的 EV,并通过单 EV 免疫荧光对 CD63 和 CD81 进行定量。尽管提取量较低,但仍观察到单个 EV 上生物标志物表达和四跨膜蛋白共表达的差异。 此外, EV μBR 能够在细胞和囊泡层面重现异质性,表明微流体系统在研究乳腺癌和其他疾病模型中生理相关 EV 方面的实用性和鲁棒性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号