Cell Metabolism ( IF 27.7 ) Pub Date : 2022-05-03 , DOI: 10.1016/j.cmet.2022.04.008 George Caputa 1 , Mai Matsushita 1 , David E Sanin 2 , Agnieszka M Kabat 1 , Joy Edwards-Hicks 1 , Katarzyna M Grzes 1 , Roland Pohlmeyer 3 , Michal A Stanczak 1 , Angela Castoldi 1 , Jovana Cupovic 1 , Aaron J Forde 4 , Petya Apostolova 1 , Maximilian Seidl 5 , Nikki van Teijlingen Bakker 6 , Matteo Villa 1 , Francesc Baixauli 1 , Andrea Quintana 1 , Alexandra Hackl 1 , Lea Flachsmann 1 , Fabian Hässler 1 , Jonathan D Curtis 1 , Annette E Patterson 1 , Philipp Henneke 7 , Erika L Pearce 8 , Edward J Pearce 9
|
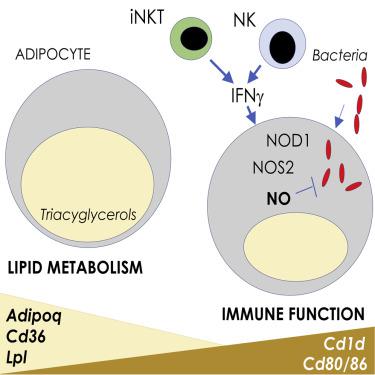
Adipose tissue (AT) plays a central role in systemic metabolic homeostasis, but its function during bacterial infection remains unclear. Following subcutaneous bacterial infection, adipocytes surrounding draining lymph nodes initiated a transcriptional response indicative of stimulation with IFN-γ and a shift away from lipid metabolism toward an immunologic function. Natural killer (NK) and invariant NK T (iNKT) cells were identified as sources of infection-induced IFN-γ in perinodal AT (PAT). IFN-γ induced Nos2 expression in adipocytes through a process dependent on nuclear-binding oligomerization domain 1 (NOD1) sensing of live intracellular bacteria. iNOS expression was coupled to metabolic rewiring, inducing increased diversion of extracellular L-arginine through the arginosuccinate shunt and urea cycle to produce nitric oxide (NO), directly mediating bacterial clearance. In vivo, control of infection in adipocytes was dependent on adipocyte-intrinsic sensing of IFN-γ and expression of iNOS. Thus, adipocytes are licensed by innate lymphocytes to acquire anti-bacterial functions during infection.
中文翻译:

细胞内感染和免疫系统提示重新连接脂肪细胞以获得免疫功能
脂肪组织 (AT) 在全身代谢稳态中发挥核心作用,但其在细菌感染期间的功能仍不清楚。皮下细菌感染后,引流淋巴结周围的脂肪细胞启动了转录反应,表明受到 IFN-γ 的刺激,并从脂质代谢转向免疫功能。自然杀伤 (NK) 和不变的 NK T (iNKT) 细胞被确定为围节期 AT (PAT) 中感染诱导的 IFN-γ 的来源。IFN-γ诱导的Nos2通过依赖于活细胞内细菌的核结合寡聚化结构域 1 (NOD1) 感应的过程在脂肪细胞中表达。iNOS 表达与代谢重新布线相结合,通过精氨酸琥珀酸分流和尿素循环诱导细胞外 L-精氨酸的转移增加,从而产生一氧化氮 (NO),直接介导细菌清除。在体内,脂肪细胞感染的控制依赖于脂肪细胞对 IFN-γ 的内在感知和 iNOS 的表达。因此,脂肪细胞被先天淋巴细胞许可在感染期间获得抗菌功能。











































 京公网安备 11010802027423号
京公网安备 11010802027423号