当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chemical Nature of Heterogeneous Electrofreezing of Supercooled Water Revealed on Polar (Pyroelectric) Surfaces
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2022-05-03 , DOI: 10.1021/acs.accounts.2c00004 Leah Fuhrman Javitt 1 , Sofia Curland 1 , Isabelle Weissbuch 1 , David Ehre 1 , Meir Lahav 1 , Igor Lubomirsky 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2022-05-03 , DOI: 10.1021/acs.accounts.2c00004 Leah Fuhrman Javitt 1 , Sofia Curland 1 , Isabelle Weissbuch 1 , David Ehre 1 , Meir Lahav 1 , Igor Lubomirsky 1
Affiliation
|
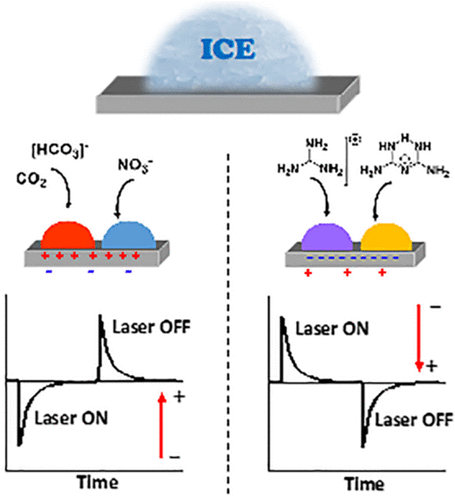
The ability to control the icing temperature of supercooled water (SCW) is of supreme importance in subfields of pure and applied sciences. The ice freezing of SCW can be influenced heterogeneously by electric effects, a process known as electrofreezing. This effect was first discovered during the 19th century; however, its mechanism is still under debate. In this Account we demonstrate, by capitalizing on the properties of polar crystals, that heterogeneous electrofreezing of SCW is a chemical process influenced by an electric field and specific ions. Polar crystals possess a net dipole moment. In addition, they are pyroelectric, displaying short-lived surface charges at their hemihedral faces at the two poles of the crystals as a result of temperature changes. Accordingly, during cooling or heating, an electric field is created, which is negated by the attraction of compensating charges from the environment. This process had an impact in the following experiments. The icing temperatures of SCW within crevices of polar crystals are higher in comparison to icing temperatures within crevices of nonpolar analogs. The role played by the electric effect was extricated from other effects by the performance of icing experiments on the surfaces of pyroelectric quasi-amorphous SrTiO3. During those studies it was found that on positively charged surfaces the icing temperature of SCW is elevated, whereas on negatively charged surfaces it is reduced. Following investigations discovered that the icing temperature of SCW is impacted by an ionic current created within a hydrated layer on top of hydrophilic faces residing parallel to the polar axes of the crystals. In the absence of such current on analogous hydrophobic surfaces, the pyroelectric effect does not influence the icing temperature of SCW. Those results implied that electrofreezing of SCW is a process influenced by specific compensating ions attracted by the pyroelectric field from the aqueous solution. When freezing experiments are performed in an open atmosphere, bicarbonate and hydronium ions, created by the dissolution of atmospheric CO2 in water, influence the icing temperature. The bicarbonate ions, when attracted by positively charged pyroelectric surfaces, elevate the icing temperature, whereas their counterparts, hydronium ions, when attracted by the negatively charged surfaces reduce the icing temperature. Molecular dynamic simulations suggested that bicarbonate ions, concentrated within the near positively charged interfacial layer, self-assemble with water molecules to create stabilized slightly distorted “ice-like” hexagonal assemblies which mimic the hexagons of the crystals of ice. This occurs by replacing, within those ice-like hexagons, two hydrogen bonds of water by C–O bonds of the HCO3– ion. On the basis of these simulations, it was predicted and experimentally confirmed that other trigonal planar ions such as NO3–, guanidinium+, and the quasi-hexagonal biguanidinium+ ion elevate the icing temperature. These ions were coined as “ice makers”. Other ions including hydronium, Cl–, and SO4–2 interfere with the formation of ice-like assemblies and operate as “ice breakers”. The higher icing temperatures induced within the crevices of the hydrophobic polar crystals in comparison to the nonpolar analogs can be attributed to the proton ordering of the water molecules. In contrast, the icing temperatures on related hydrophilic surfaces are influenced both by compensating charges and by proton ordering.
中文翻译:

极性(热释电)表面揭示的过冷水非均相电冷冻的化学性质
控制过冷水结冰温度 (SCW) 的能力在纯科学和应用科学的子领域中至关重要。SCW 的冰冻结会受到电效应的不同影响,这一过程称为电冻结。这种效应最早是在 19 世纪发现的。然而,其机制仍在争论中。在这个帐户中,我们通过利用极性晶体的特性证明 SCW 的异质电冷冻是受电场和特定离子影响的化学过程。极性晶体具有净偶极矩。此外,它们是热释电的,由于温度变化,它们在晶体的两个极的半面体面上显示出短暂的表面电荷。因此,在冷却或加热过程中,会产生电场,这被来自环境的补偿电荷的吸引所抵消。这个过程对后面的实验产生了影响。与非极性晶体缝隙中的结冰温度相比,极性晶体缝隙中 SCW 的结冰温度更高。通过在热释电准非晶 SrTiO 表面进行结冰实验,将电效应所起的作用从其他效应中解脱出来3 . 在这些研究中发现,在带正电的表面上,SCW 的结冰温度升高,而在带负电的表面上降低。随后的研究发现,SCW 的结冰温度受到在平行于晶体极轴的亲水面顶部的水合层内产生的离子电流的影响。在类似疏水表面上没有这种电流的情况下,热电效应不会影响 SCW 的结冰温度。这些结果表明SCW的电冷冻是一个受水溶液中热电场吸引的特定补偿离子影响的过程。当在开放大气中进行冷冻实验时,由大气 CO 溶解产生的碳酸氢盐和水合氢离子2在水中,影响结冰温度。碳酸氢根离子在被带正电的热释电表面吸引时会提高结冰温度,而它们的对应物水合氢离子在被带负电的表面吸引时会降低结冰温度。分子动力学模拟表明,碳酸氢根离子集中在接近带正电的界面层内,与水分子自组装,形成稳定的、略微扭曲的“冰状”六角形组件,模拟冰晶体的六角形。这是通过在那些冰状六边形中,用 HCO 3的 C-O 键取代水的两个氢键来实现的-离子。在这些模拟的基础上,预测和实验证实了其他三角平面离子如 NO 3 -、胍+和准六方双胍+离子会提高结冰温度。这些离子被称为“制冰机”。其他离子,包括水合氢离子、Cl –和 SO 4 –2干扰冰状组件的形成并作为“破冰船”运行。与非极性类似物相比,在疏水极性晶体的缝隙中诱导的较高结冰温度可归因于水分子的质子排序。相反,相关亲水表面上的结冰温度受补偿电荷和质子排序的影响。
更新日期:2022-05-03
中文翻译:

极性(热释电)表面揭示的过冷水非均相电冷冻的化学性质
控制过冷水结冰温度 (SCW) 的能力在纯科学和应用科学的子领域中至关重要。SCW 的冰冻结会受到电效应的不同影响,这一过程称为电冻结。这种效应最早是在 19 世纪发现的。然而,其机制仍在争论中。在这个帐户中,我们通过利用极性晶体的特性证明 SCW 的异质电冷冻是受电场和特定离子影响的化学过程。极性晶体具有净偶极矩。此外,它们是热释电的,由于温度变化,它们在晶体的两个极的半面体面上显示出短暂的表面电荷。因此,在冷却或加热过程中,会产生电场,这被来自环境的补偿电荷的吸引所抵消。这个过程对后面的实验产生了影响。与非极性晶体缝隙中的结冰温度相比,极性晶体缝隙中 SCW 的结冰温度更高。通过在热释电准非晶 SrTiO 表面进行结冰实验,将电效应所起的作用从其他效应中解脱出来3 . 在这些研究中发现,在带正电的表面上,SCW 的结冰温度升高,而在带负电的表面上降低。随后的研究发现,SCW 的结冰温度受到在平行于晶体极轴的亲水面顶部的水合层内产生的离子电流的影响。在类似疏水表面上没有这种电流的情况下,热电效应不会影响 SCW 的结冰温度。这些结果表明SCW的电冷冻是一个受水溶液中热电场吸引的特定补偿离子影响的过程。当在开放大气中进行冷冻实验时,由大气 CO 溶解产生的碳酸氢盐和水合氢离子2在水中,影响结冰温度。碳酸氢根离子在被带正电的热释电表面吸引时会提高结冰温度,而它们的对应物水合氢离子在被带负电的表面吸引时会降低结冰温度。分子动力学模拟表明,碳酸氢根离子集中在接近带正电的界面层内,与水分子自组装,形成稳定的、略微扭曲的“冰状”六角形组件,模拟冰晶体的六角形。这是通过在那些冰状六边形中,用 HCO 3的 C-O 键取代水的两个氢键来实现的-离子。在这些模拟的基础上,预测和实验证实了其他三角平面离子如 NO 3 -、胍+和准六方双胍+离子会提高结冰温度。这些离子被称为“制冰机”。其他离子,包括水合氢离子、Cl –和 SO 4 –2干扰冰状组件的形成并作为“破冰船”运行。与非极性类似物相比,在疏水极性晶体的缝隙中诱导的较高结冰温度可归因于水分子的质子排序。相反,相关亲水表面上的结冰温度受补偿电荷和质子排序的影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号