当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chiral Posttranslational Modification to Lysine ε-Amino Groups
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2022-05-02 , DOI: 10.1021/acs.accounts.2c00115 Carlos Moreno-Yruela 1 , Michael Bæk 1 , Fabrizio Monda 1 , Christian A Olsen 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2022-05-02 , DOI: 10.1021/acs.accounts.2c00115 Carlos Moreno-Yruela 1 , Michael Bæk 1 , Fabrizio Monda 1 , Christian A Olsen 1
Affiliation
|
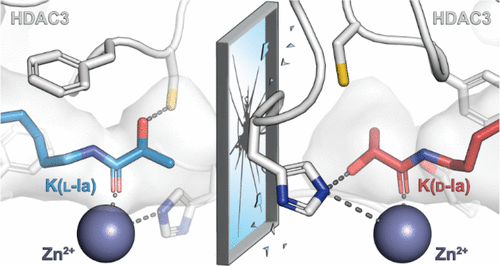
The sophistication of proteomic analysis has revealed that protein lysine residues are posttranslationally modified by a variety of acyl groups. Protein lysine acetylation regulates metabolism, gene expression, and microtubule formation and has been extensively studied; however, the understanding of the biological significance of other acyl posttranslational modifications (PTMs) is still in its infancy. The acylation of lysine residues is mediated either by acyltransferase “writer” enzymes or by nonenzymatic mechanisms and hydrolase enzymes, termed “erasers”, that cleave various acyl PTMs to reverse the modified state. We have studied the human lysine deacylase enzymes, comprising the 11 Zn2+-dependent histone deacetylases (HDACs) and the 7 NAD+-consuming sirtuins (SIRTs), over the past decade. We have thus developed selective inhibitors and molecular probes and have studied the acyl substrate scope of each enzyme using chemically synthesized peptide substrates and photo-cross-linking probes. Recently, we have turned our attention to PTMs containing a stereogenic center, such as ε-N-β-hydroxybutyryllysine (Kbhb) and ε-N-lactyllysine (Kla), that each comprise a pair of mirror image stereoisomers as modifications. Both modifications are found on histones, where they affect gene transcription in response to specific metabolic states, and they are found on cytosolic and mitochondrial enzymes involved in fatty acid oxidation (Kbhb) and glycolysis (Kla), respectively. Thus, chiral modifications to lysine side chains give rise to two distinct diastereomeric products, with separate metabolic origins and potentially different activities exhibited by writer and eraser enzymes. Lysine l-lactylation originates from l-lactate, a major energy carrier produced from pyruvate after glycolysis, and it is highly induced by metabolic states such as the Warburg effect. l-Lactate can possibly be activated by acyl-coenzyme A (CoA) synthetases and transferred to lysine residues by histone acetyltransferases such as p300. d-Lactylation, on the other hand, arises primarily from a nonenzymatic reaction with d-lactylglutathione, an intermediate in the glyoxalase pathway. In addition to their distinct origin, we found that both K(l-la) and K(d-la) modifications are erased by HDACs with different catalytic efficiencies. Also, K(l-bhb) and K(d-bhb) arise from different metabolites but depend on interconnected metabolic pathways, and the two stereoisomers of ε-N-3-hydroxy-3-methylglutaryllysine (Khmg) originate from a single precursor that may then be regulated differently by eraser enzymes. Distinguishing between the individual stereoisomers of PTMs is therefore of crucial importance. In the present Account, we will (1) revisit the long-standing evidence for the distinct production and dynamics of enantiomeric forms of chiral metabolites that serve as ε-N-acyllysine PTMs and (2) highlight the outstanding questions that arise from the recent literature on chiral lysine PTMs resulting from these metabolites.
中文翻译:

赖氨酸 ε-氨基的手性翻译后修饰
蛋白质组学分析的复杂性表明蛋白质赖氨酸残基在翻译后被多种酰基修饰。蛋白质赖氨酸乙酰化调节代谢、基因表达和微管形成,并已被广泛研究;然而,对其他酰基翻译后修饰 (PTM) 的生物学意义的理解仍处于起步阶段。赖氨酸残基的酰化由酰基转移酶“作家”酶或非酶机制和水解酶介导,称为“橡皮擦”,可切割各种酰基 PTM 以逆转修饰状态。我们研究了人类赖氨酸脱乙酰酶,包括 11 个 Zn 2+依赖性组蛋白脱乙酰酶 (HDAC) 和 7 个 NAD +- 在过去十年中消耗了sirtuins (SIRTs)。因此,我们开发了选择性抑制剂和分子探针,并使用化学合成的肽底物和光交联探针研究了每种酶的酰基底物范围。最近,我们将注意力转向含有立体中心的 PTM,例如ε- N -β-羟基丁酰赖氨酸 (Kbhb) 和 ε- N-乳酰赖氨酸(Kla),每个都包含一对镜像立体异构体作为修饰。这两种修饰都存在于组蛋白上,它们会影响基因转录以响应特定的代谢状态,并且它们分别存在于参与脂肪酸氧化 (Kbhb) 和糖酵解 (Kla) 的胞质酶和线粒体酶上。因此,对赖氨酸侧链的手性修饰会产生两种不同的非对映体产物,它们具有不同的代谢来源,并且可能由写入酶和擦除酶表现出不同的活性。赖氨酸L-乳酸化来源于L-乳酸,L-乳酸是丙酮酸在糖酵解后产生的主要能量载体,并且受到Warburg效应等代谢状态的高度诱导。l-乳酸可能被酰基辅酶 A (CoA) 合成酶激活,并被组蛋白乙酰转移酶如 p300 转移到赖氨酸残基上。另一方面,d-乳酸化主要来自与乙二醛酶途径中的中间体d-乳酰谷胱甘肽的非酶反应。除了它们不同的起源之外,我们发现 K( l -la) 和 K( d -la) 修饰都被具有不同催化效率的 HDAC 擦除。此外,K( l -bhb) 和 K( d -bhb) 来自不同的代谢物,但依赖于相互关联的代谢途径,以及 ε- N的两种立体异构体-3-羟基-3-甲基戊二酰赖氨酸 (Khmg) 源自单一前体,然后可能受到橡皮酶的不同调节。因此,区分 PTM 的各个立体异构体至关重要。在本报告中,我们将 (1) 重新审视作为 ε- N - 酰基赖氨酸 PTM的对映体形式的手性代谢物的独特产生和动力学的长期证据,以及 (2) 强调最近出现的悬而未决的问题有关由这些代谢物产生的手性赖氨酸 PTM 的文献。
更新日期:2022-05-02
中文翻译:

赖氨酸 ε-氨基的手性翻译后修饰
蛋白质组学分析的复杂性表明蛋白质赖氨酸残基在翻译后被多种酰基修饰。蛋白质赖氨酸乙酰化调节代谢、基因表达和微管形成,并已被广泛研究;然而,对其他酰基翻译后修饰 (PTM) 的生物学意义的理解仍处于起步阶段。赖氨酸残基的酰化由酰基转移酶“作家”酶或非酶机制和水解酶介导,称为“橡皮擦”,可切割各种酰基 PTM 以逆转修饰状态。我们研究了人类赖氨酸脱乙酰酶,包括 11 个 Zn 2+依赖性组蛋白脱乙酰酶 (HDAC) 和 7 个 NAD +- 在过去十年中消耗了sirtuins (SIRTs)。因此,我们开发了选择性抑制剂和分子探针,并使用化学合成的肽底物和光交联探针研究了每种酶的酰基底物范围。最近,我们将注意力转向含有立体中心的 PTM,例如ε- N -β-羟基丁酰赖氨酸 (Kbhb) 和 ε- N-乳酰赖氨酸(Kla),每个都包含一对镜像立体异构体作为修饰。这两种修饰都存在于组蛋白上,它们会影响基因转录以响应特定的代谢状态,并且它们分别存在于参与脂肪酸氧化 (Kbhb) 和糖酵解 (Kla) 的胞质酶和线粒体酶上。因此,对赖氨酸侧链的手性修饰会产生两种不同的非对映体产物,它们具有不同的代谢来源,并且可能由写入酶和擦除酶表现出不同的活性。赖氨酸L-乳酸化来源于L-乳酸,L-乳酸是丙酮酸在糖酵解后产生的主要能量载体,并且受到Warburg效应等代谢状态的高度诱导。l-乳酸可能被酰基辅酶 A (CoA) 合成酶激活,并被组蛋白乙酰转移酶如 p300 转移到赖氨酸残基上。另一方面,d-乳酸化主要来自与乙二醛酶途径中的中间体d-乳酰谷胱甘肽的非酶反应。除了它们不同的起源之外,我们发现 K( l -la) 和 K( d -la) 修饰都被具有不同催化效率的 HDAC 擦除。此外,K( l -bhb) 和 K( d -bhb) 来自不同的代谢物,但依赖于相互关联的代谢途径,以及 ε- N的两种立体异构体-3-羟基-3-甲基戊二酰赖氨酸 (Khmg) 源自单一前体,然后可能受到橡皮酶的不同调节。因此,区分 PTM 的各个立体异构体至关重要。在本报告中,我们将 (1) 重新审视作为 ε- N - 酰基赖氨酸 PTM的对映体形式的手性代谢物的独特产生和动力学的长期证据,以及 (2) 强调最近出现的悬而未决的问题有关由这些代谢物产生的手性赖氨酸 PTM 的文献。











































 京公网安备 11010802027423号
京公网安备 11010802027423号