Journal of Catalysis ( IF 6.5 ) Pub Date : 2022-04-29 , DOI: 10.1016/j.jcat.2022.04.027 Yangyu Zhang 1 , Mingyuan Zhang 1 , Yanliang Zhou 1 , Linlin Yang 1 , Bingyu Lin 1 , Jun Ni 1 , Lirong Zheng 2 , Xiuyun Wang 1 , Chak-tong Au 1 , Lilong Jiang 1
|
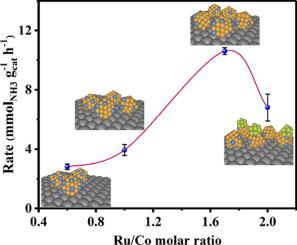
Rational design of efficient catalysts for ammonia (NH3) synthesis at mild conditions is of paramount importance for the development of electrolysis driven Haber-Bosch (eHB) process. Ammonia synthesis is a structure sensitive reaction, and any small change of electronic structure could result in dramatic change of catalytic activity, especially for the Ru-based NH3 synthesis catalysts. Electronic structure modulation of the Ru-based catalysts could provide desirable catalytic activity. Herein, we explore a class of Ru-Co single-atom alloy catalysts (marked as RuxCo1 SAA, x stands for Ru/Co molar ratio), in which Co single atoms are located on Ru nanoclusters to form Co-Ru coordination, and then electronic structure of RuxCo1 SAA could be effectively tuned by regulating the Ru/Co molar ratio. Our studies show that a moderate ratio of Ru/Co (1.7:1) favors superior NH3 synthesis rate and low activation energy. Using a suite of elaborate characterizations, we have observed that the outstanding performance over Ru1.7Co1 SAA can be attributed to the electron redistribution between Ru and Co atom, resulting in an upshift of d band center and lowering of work function. However, there is decrease of NH3 synthesis rate when Ru/Co molar ratio is 2, due to the partial detachment of Ru entities from SAA structure, and the formed individual Ru nanoparticles (NPs) overlay the surface of catalyst. In such a case, N2 activation behavior over Ru2Co1 is alike that of Ru NPs catalysts. The present study demonstrates that the Ru/Co ratio in SAA structure can effectively regulate the electronic structure of catalyst for NH3 synthesis rate enhancement.
中文翻译:

深入了解Ru和Co之间强相互作用在RuCo单原子合金结构中对显着提高氨合成性能的关键作用
在温和条件下合理设计用于氨 (NH 3 ) 合成的高效催化剂对于开发电解驱动的 Haber-Bosch ( e HB) 工艺至关重要。氨合成是一种结构敏感的反应,电子结构的任何微小变化都会导致催化活性的剧烈变化,尤其是对于Ru基NH 3合成催化剂。Ru基催化剂的电子结构调节可以提供理想的催化活性。在此,我们探索了一类Ru-Co单原子合金催化剂(标记为Ru x Co 1SAA,x代表Ru/Co摩尔比),其中Co单原子位于Ru纳米团簇上形成Co-Ru配位,然后通过调节Ru/Co摩尔比可以有效调节Ru x Co 1 SAA的电子结构比率。我们的研究表明,适度的 Ru/Co 比 (1.7:1) 有利于出色的 NH 3合成速率和低活化能。通过一系列详尽的表征,我们观察到 Ru 1.7 Co 1 SAA 的出色性能可归因于 Ru 和 Co 原子之间的电子重新分布,导致d带中心上移和功函数降低。然而,NH 3减少当Ru/Co摩尔比为2时,由于Ru实体部分从SAA结构中脱离,形成的单个Ru纳米颗粒(NPs)覆盖在催化剂表面,从而提高了合成速率。在这种情况下, Ru 2 Co 1上的 N 2活化行为与 Ru NPs 催化剂相似。本研究表明,SAA结构中的Ru/Co比可以有效调节催化剂的电子结构,从而提高NH 3合成速率。











































 京公网安备 11010802027423号
京公网安备 11010802027423号