当前位置:
X-MOL 学术
›
ACS Macro Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Morphological Selectivity of a Protein Self-Assembly System with a Repertoire of Diverse Interaction Modes
ACS Macro Letters ( IF 5.1 ) Pub Date : 2022-04-29 , DOI: 10.1021/acsmacrolett.2c00154 Xiumei Li 1, 2 , Yushi Bai 3 , Quan Luo 4 , Jiayun Xu 2 , Tianfeng Chen 1 , Junqiu Liu 2
ACS Macro Letters ( IF 5.1 ) Pub Date : 2022-04-29 , DOI: 10.1021/acsmacrolett.2c00154 Xiumei Li 1, 2 , Yushi Bai 3 , Quan Luo 4 , Jiayun Xu 2 , Tianfeng Chen 1 , Junqiu Liu 2
Affiliation
|
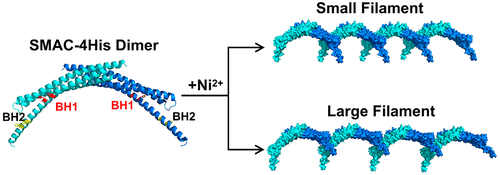
Multiple metal chelating sites were incorporated onto the second mitochondria-derived activator of caspase (SMAC) building blocks. The combination of different binding sites generated a repertoire of diverse binding modes, among which two different microfilament types (small and large) with distinct patterns were selected under thermodynamic control. Furthermore, the two microfilaments exhibited a pronounced secondary assembly trend due to the potential noncovalent interactions on the protein surfaces. Coupled with stereoselectivity, they presented a strong self-recognition effect and underwent two distinct reassembly patterns. That is, the large filaments self-associated in pairs to form “interlocked chain” structures, while the small ones twisted to form protein helical bundles. This work represents one of the few studies of selective self-assembly of self-assembled protein assemblies. Such an idea may provide inspiration for constructing more sophisticated protein architectures in the future.
中文翻译:

具有多种相互作用模式的蛋白质自组装系统的形态选择性
多个金属螯合位点被整合到第二个线粒体衍生的半胱天冬酶激活剂 (SMAC) 构建块上。不同结合位点的组合产生了多种结合模式,其中在热力学控制下选择了两种具有不同模式的不同微丝类型(小和大)。此外,由于蛋白质表面潜在的非共价相互作用,两条微丝表现出明显的二次组装趋势。再加上立体选择性,它们呈现出强烈的自我识别效果,并经历了两种不同的重组模式。也就是说,大细丝成对自结合形成“互锁链”结构,而小细丝则扭曲形成蛋白质螺旋束。这项工作代表了自组装蛋白质组装体的选择性自组装的少数研究之一。这样的想法可能会为未来构建更复杂的蛋白质结构提供灵感。
更新日期:2022-04-29
中文翻译:

具有多种相互作用模式的蛋白质自组装系统的形态选择性
多个金属螯合位点被整合到第二个线粒体衍生的半胱天冬酶激活剂 (SMAC) 构建块上。不同结合位点的组合产生了多种结合模式,其中在热力学控制下选择了两种具有不同模式的不同微丝类型(小和大)。此外,由于蛋白质表面潜在的非共价相互作用,两条微丝表现出明显的二次组装趋势。再加上立体选择性,它们呈现出强烈的自我识别效果,并经历了两种不同的重组模式。也就是说,大细丝成对自结合形成“互锁链”结构,而小细丝则扭曲形成蛋白质螺旋束。这项工作代表了自组装蛋白质组装体的选择性自组装的少数研究之一。这样的想法可能会为未来构建更复杂的蛋白质结构提供灵感。











































 京公网安备 11010802027423号
京公网安备 11010802027423号