当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Local reaction environment for selective electroreduction of carbon monoxide
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2022-04-28 , DOI: 10.1039/d1ee03838a Ming Ma 1, 2 , Wanyu Deng 2 , Aoni Xu 2 , Degenhart Hochfilzer 2 , Yu Qiao 2 , Karen Chan 2 , Ib Chorkendorff 2 , Brian Seger 2
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2022-04-28 , DOI: 10.1039/d1ee03838a Ming Ma 1, 2 , Wanyu Deng 2 , Aoni Xu 2 , Degenhart Hochfilzer 2 , Yu Qiao 2 , Karen Chan 2 , Ib Chorkendorff 2 , Brian Seger 2
Affiliation
|
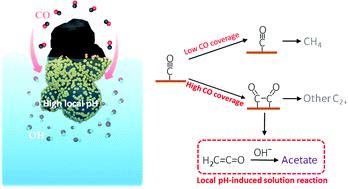
The electroreduction of CO2 to multi-carbon products (C2+) is an attractive route for high-density renewable energy storage, but catalytic selectivity toward a desired C2+ product is low. Although the pH in the vicinity of the catalysts has previously been correlated with selectivity of C2+ products, solid evidence of the local pH effect on specific C2+ products is lacking. Here, we present a very simple strategy for experimentally probing the local pH value in GDE-based high-rate CO electroreduction via the CO2 capture rate. From the local pH determination method as well as the consideration of other possibilities that may influence the C2+ formation, we explicitly demonstrate that the increased OH− concentration near the cathodic GDE surface in CO reduction can significantly facilitate acetate formation via a homogeneous solution reaction which is the key step, influenced by local pH near the cathode surface, correspondingly inhibiting other C2+ products such as ethanol, n-propanol and C2H4. Notably, a total C2+ faradaic efficiency of >90% that encompasses up to nearly 50% acetate faradaic efficiency is achieved via elevating average local OH− concentration. In addition, the exploration of the link between local CO availability and the specific C2+ selectivity demonstrates that high CO coverage is required for C2+ formation owing to a preferred CO coupling. Further analysis indicates that a certain intermediate should be directly shared for acetate and ethanol, but the relevant reaction paths of this intermediate may be separated from that of C2H4.
中文翻译:

一氧化碳选择性电还原的局部反应环境
将CO 2电还原为多碳产物(C 2+)是高密度可再生能源存储的有吸引力的途径,但对所需C 2+产物的催化选择性较低。尽管催化剂附近的 pH 值以前与 C 2+ 产物的选择性相关,但缺乏对特定 C 2+产物的局部 pH 影响的可靠证据。在这里,我们提出了一种非常简单的策略,用于通过CO 2捕获率对基于 GDE 的高速 CO 电还原中的局部 pH 值进行实验性探测。从当地的 pH 测定方法以及可能影响 C 2+的其他可能性的考虑形成,我们明确地证明,在 CO 还原过程中,阴极 GDE 表面附近OH -浓度的增加可以通过均相溶液反应显着促进乙酸盐的形成,这是关键步骤,受阴极表面附近局部 pH 值的影响,相应地抑制了其他 C 2+乙醇、正丙醇、C 2 H 4等产品。值得注意的是,通过提高平均局部 OH - 实现了大于 90%的总 C 2+法拉第效率,其中包括高达近 50% 的醋酸法拉第效率-浓度。此外,对局部 CO 可用性和特定 C 2+选择性之间联系的探索表明,由于优选的 CO 偶联,C 2+形成需要高 CO 覆盖率。进一步分析表明,乙酸和乙醇应该直接共用某个中间体,但该中间体的相关反应路径可能与C 2 H 4的反应路径分开。
更新日期:2022-04-28
中文翻译:

一氧化碳选择性电还原的局部反应环境
将CO 2电还原为多碳产物(C 2+)是高密度可再生能源存储的有吸引力的途径,但对所需C 2+产物的催化选择性较低。尽管催化剂附近的 pH 值以前与 C 2+ 产物的选择性相关,但缺乏对特定 C 2+产物的局部 pH 影响的可靠证据。在这里,我们提出了一种非常简单的策略,用于通过CO 2捕获率对基于 GDE 的高速 CO 电还原中的局部 pH 值进行实验性探测。从当地的 pH 测定方法以及可能影响 C 2+的其他可能性的考虑形成,我们明确地证明,在 CO 还原过程中,阴极 GDE 表面附近OH -浓度的增加可以通过均相溶液反应显着促进乙酸盐的形成,这是关键步骤,受阴极表面附近局部 pH 值的影响,相应地抑制了其他 C 2+乙醇、正丙醇、C 2 H 4等产品。值得注意的是,通过提高平均局部 OH - 实现了大于 90%的总 C 2+法拉第效率,其中包括高达近 50% 的醋酸法拉第效率-浓度。此外,对局部 CO 可用性和特定 C 2+选择性之间联系的探索表明,由于优选的 CO 偶联,C 2+形成需要高 CO 覆盖率。进一步分析表明,乙酸和乙醇应该直接共用某个中间体,但该中间体的相关反应路径可能与C 2 H 4的反应路径分开。











































 京公网安备 11010802027423号
京公网安备 11010802027423号