当前位置:
X-MOL 学术
›
Curr. Drug Deliv.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Macrophage-Mediated Delivery of Fe3O4-Nanoparticles: A Generalized Strategy to Deliver Iron to Tumor Microenvironment
Current Drug Delivery ( IF 2.8 ) Pub Date : 2022-04-27 , DOI: 10.2174/1567201819666220426085450 Cong Wu 1, 2, 3 , Guozhong Zhang 1, 2, 3 , Zhihao Wang 1, 2, 3 , Hongcan Shi 1, 2, 3
Current Drug Delivery ( IF 2.8 ) Pub Date : 2022-04-27 , DOI: 10.2174/1567201819666220426085450 Cong Wu 1, 2, 3 , Guozhong Zhang 1, 2, 3 , Zhihao Wang 1, 2, 3 , Hongcan Shi 1, 2, 3
Affiliation
|
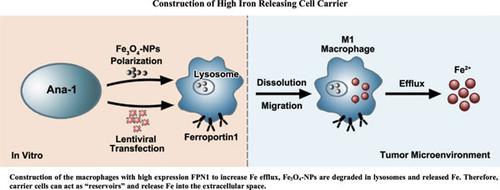
Background: Iron is used to alter macrophage phenotypes and induce tumor cell death. Iron oxide nanoparticles can induce macrophage polarization into the M1 phenotype, which inhibits tumor growth and can dissociate into iron ions in macrophages. Objective: In this study, we proposed to construct high expression of Ferroportin1 macrophages as carriers to deliver Fe3O4-nanoparticles and iron directly to tumor sites. Methods: Three sizes of Fe3O4-nanoparticles with gradient concentrations were used. The migration ability of iron-carrying macrophages was confirmed by an in vitro migration experiment and monocyte chemoattractant protein-1 detection. The release of iron from macrophages was confirmed by determining their levels in the cell culture supernatant, and we constructed a high expression of ferroportin strain of macrophage lines to increase intracellular iron efflux by increasing membrane transferrin expression. Fe3O4-NPs in Ana-1 cells were degraded in lysosomes, and the amount of iron released was correlated with the expression of ferroportin1. Results: After Fe3O4-nanoparticles uptake by macrophages, not only polarized macrophages into M1 phenotype, but the nanoparticles also dissolved in the lysosome and iron were released out of the cell. FPN1 is the only known Fe transporter; we use a Lentiviral vector carrying the FPN1 gene transfected into macrophages, has successfully constructed Ana-1-FPN1 cells, and maintains high expression of FPN1. Ana-1-FPN1 cells increase intracellular iron release. Fe3O4-nanoparticles loaded with engineered Ana-1 macrophages can act as a “reservoir” of iron. Conclusion: Our study provides proof of strategy for Fe3O4-NPs target delivery to the tumor microenvironment. Moreover, increase of intracellular iron efflux by overexpression of FPN1, cell carriers can act as a reservoir for iron, providing the basis for targeted delivery of Fe3O4-NPs and iron ions in vivo.
中文翻译:

巨噬细胞介导的 Fe3O4 纳米颗粒递送:将铁递送至肿瘤微环境的通用策略
背景:铁用于改变巨噬细胞表型并诱导肿瘤细胞死亡。氧化铁纳米粒子可诱导巨噬细胞极化为 M1 表型,从而抑制肿瘤生长并可在巨噬细胞中解离成铁离子。目的:在本研究中,我们提出构建高表达 Ferroportin1 巨噬细胞作为载体,将 Fe3O4 纳米颗粒和铁直接递送至肿瘤部位。方法:使用具有梯度浓度的三种尺寸的 Fe3O4 纳米颗粒。通过体外迁移实验和单核细胞趋化蛋白-1检测证实了携带铁的巨噬细胞的迁移能力。铁从巨噬细胞中的释放通过测定它们在细胞培养上清液中的水平来确认,我们构建了高表达铁转运蛋白的巨噬细胞系菌株,通过增加膜转铁蛋白表达来增加细胞内铁流出。Ana-1 细胞中的 Fe3O4-NPs 在溶酶体中被降解,释放的铁量与铁转运蛋白 1 的表达相关。结果:Fe3O4-纳米颗粒被巨噬细胞摄取后,不仅巨噬细胞极化为M1表型,而且纳米颗粒也溶解在溶酶体中,铁被释放出细胞。FPN1 是唯一已知的 Fe 转运蛋白;我们使用携带FPN1基因的慢病毒载体转染巨噬细胞,成功构建了Ana-1-FPN1细胞,并保持了FPN1的高表达。Ana-1-FPN1 细胞增加细胞内铁释放。载有工程 Ana-1 巨噬细胞的 Fe3O4 纳米颗粒可以充当铁的“储库”。结论:我们的研究为将 Fe3O4-NPs 靶向递送至肿瘤微环境提供了策略证据。此外,通过 FPN1 的过表达增加细胞内铁流出,细胞载体可以充当铁的储库,为体内靶向递送 Fe3O4-NPs 和铁离子提供基础。
更新日期:2022-04-27
中文翻译:

巨噬细胞介导的 Fe3O4 纳米颗粒递送:将铁递送至肿瘤微环境的通用策略
背景:铁用于改变巨噬细胞表型并诱导肿瘤细胞死亡。氧化铁纳米粒子可诱导巨噬细胞极化为 M1 表型,从而抑制肿瘤生长并可在巨噬细胞中解离成铁离子。目的:在本研究中,我们提出构建高表达 Ferroportin1 巨噬细胞作为载体,将 Fe3O4 纳米颗粒和铁直接递送至肿瘤部位。方法:使用具有梯度浓度的三种尺寸的 Fe3O4 纳米颗粒。通过体外迁移实验和单核细胞趋化蛋白-1检测证实了携带铁的巨噬细胞的迁移能力。铁从巨噬细胞中的释放通过测定它们在细胞培养上清液中的水平来确认,我们构建了高表达铁转运蛋白的巨噬细胞系菌株,通过增加膜转铁蛋白表达来增加细胞内铁流出。Ana-1 细胞中的 Fe3O4-NPs 在溶酶体中被降解,释放的铁量与铁转运蛋白 1 的表达相关。结果:Fe3O4-纳米颗粒被巨噬细胞摄取后,不仅巨噬细胞极化为M1表型,而且纳米颗粒也溶解在溶酶体中,铁被释放出细胞。FPN1 是唯一已知的 Fe 转运蛋白;我们使用携带FPN1基因的慢病毒载体转染巨噬细胞,成功构建了Ana-1-FPN1细胞,并保持了FPN1的高表达。Ana-1-FPN1 细胞增加细胞内铁释放。载有工程 Ana-1 巨噬细胞的 Fe3O4 纳米颗粒可以充当铁的“储库”。结论:我们的研究为将 Fe3O4-NPs 靶向递送至肿瘤微环境提供了策略证据。此外,通过 FPN1 的过表达增加细胞内铁流出,细胞载体可以充当铁的储库,为体内靶向递送 Fe3O4-NPs 和铁离子提供基础。











































 京公网安备 11010802027423号
京公网安备 11010802027423号