Agricultural and Forest Entomology ( IF 1.6 ) Pub Date : 2022-04-27 , DOI: 10.1111/afe.12504 Jordan P. Cuff 1 , Sharon Aifionn Evans 1 , Ian Angel Porteous 1 , Julia Quiñonez 1 , Darren M. Evans 1
|
INTRODUCTION
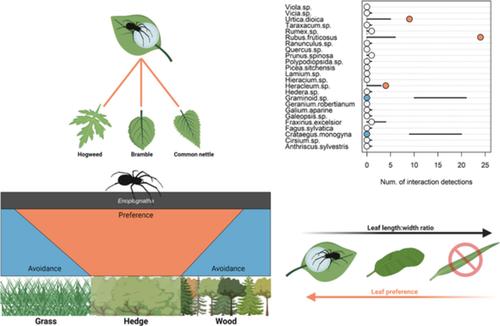
The candy-striped spiders Enoplognatha ovata (Clerck, 1757; Araneae: Theridiidae) and E. latimana Hippa & Oksala, 1982 are among the commonest theridiid spiders in the British Isles. In late summer, candy-striped spiders mate, the male dying soon after, while the female rolls live leaves with silk (Eberhard et al., 2008) in which their characteristic blue egg sac is deposited and then guarded until shortly before the second instar spiderlings emerge from the leaf-roll (Oxford, 1993; Figure 1). The number of mature female candy-striped spiders with egg sacs is thought to vary greatly depending on climate, the availability of suitable habitat, disturbance and other factors, resulting in 4 to 55-fold population fluxes over time (Oxford, 1993). In Britain, populations of up to 2744 E. ovata have been recorded from a single broad-leaved vegetation clump (Oxford, 1993) and estimates of up to 0.5 adult or 2 juvenile Enoplognatha spp. spiders were recorded per square metre in a wheat field in arid shrubland (Opatovsky et al., 2016). Populations will, however, likely vary greatly between regions and patches based on habitat suitability. Leaf-rolls such as those constructed for egg deposition by candy-striped spiders confer significant benefits to immature arthropods such as protection from parasitism and egg predation (Kobayashi et al., 2020; Loeffler, 1996; Tvardikova & Novotny, 2012). These structures can, however, introduce additional predation pressure from visually-oriented predators (Danthanarayana, 1983; Kobayashi et al., 2020) or inadvertent vertebrate herbivores through accidental consumption (Tercel et al., 2021).

The presence of candy-striped spider egg sacs in these leaf rolls is predicated on the presence of suitable plant species. In an agricultural context, this means that their potential biological control of crop pests is dependent on the floral community composition of semi-natural habitats adjacent to crop fields. E. ovata have long been known to exploit the leaves of bramble, but interactions with those of rosebay willowherb, elm, maple, hazel, ash, beech, willow, oak, may, nettles, umbellifers, Convolvulus and various other plants have also been recorded (Bristowe, 1931; Oxford, 1993). These preferences seemingly extend beyond taxonomy and may depend on leaf traits (Zanatta et al., 2022); for example, Stevenson and Dindal (1982) found more juvenile E. ovata on curled leaves than on flat, although this was in leaf litter rather than live leaves. The extension of this preference to plant species, and how it factors into egg deposition, is, however, poorly characterized. Although some plant species are known to be used more than others (e.g., common nettle Urtica dioica L., bramble Rubus fruticosus L.; Bristowe, 1931; Oxford, 1993), the preferences of candy-striped spiders for these in mixed plant communities, and the suitability of alternatives, are poorly understood.
Although theridiids such as candy-striped spiders are often found in or near agricultural crops, they are nonagrobiont spiders (i.e., they do not complete their life cycle in the crop itself) but nonetheless significantly contribute to the web-building spider populations of many agroecosystems (Gavish-Regev et al., 2008; Pluess et al., 2008). Enoplognatha ovata has been scarcely studied as a biocontrol agent in Britain, but molecular analysis has revealed that these spiders frequently predate pests such as leafhoppers in British crops (Virant-Doberlet et al., 2011). Globally, other species within the Enoplognatha genus have been studied as biocontrol agents of pest beetles (Ranjbar Aghdam, 2013; Tóth et al., 2002) lepidopterans (Luo et al., 2014), aphids (Gavish-Regev et al., 2009) and planthoppers (Chiu, 1979; Ganeshan, 2013). Enoplognatha spiders immigrate into crop fields from adjacent habitats and exhibit a high degree of preference toward pests such as aphids (Opatovsky et al., 2012). For candy-striped spiders to enter crops as immigrant biocontrol agents, they first need to be established in adjacent semi-natural habitats, contingent on the provision of suitable habitat and host plants.
By studying the interactions between candy-striped spiders and their host plants and different semi-natural habitats, it is possible to elucidate their preferences, which will have profound implications for their ecology and potential for biocontrol. Such analyses are possible through the lens of network ecology, which is the characterization and assessment of interactions between communities of organisms in complex ecological systems (Bascompte, 2007). Empirical network ecology often uses observations of species interactions in the field to construct interaction networks, which are a powerful means for assessing animal choice in situ (Cuff, Windsor, et al., 2022; Vaughan et al., 2018). The interactions between animals and plants are, however, mostly studied in antagonistic (e.g., herbivory) or mutualistic (e.g., seed dispersal, pollination) contexts, and not in the commensal context of leaf-rolling by largely predatory animals.
In this study, we apply the principles of network ecology and null model-based choice analysis to observations of candy-striped spider leaf and habitat choice in northeast England to improve our understanding of the preferences of these spiders. We test the following hypotheses: (i) candy-striped spiders disproportionately utilize hedgerow habitats for egg deposition given their greater diversity of shrub species; (ii) candy-striped spiders exhibit preference for a small range of common plant species when depositing eggs; and (iii) the plants selected by candy-striped spiders for egg deposition share similar leaf morphology. By better understanding the microhabitat preferences of these spiders, agricultural management and semi-natural habitat availability can be adjusted to ensure proliferation of these potentially important biocontrol agents.
中文翻译:

糖果条纹蜘蛛叶和产卵的栖息地偏好
介绍
糖果条纹蜘蛛Enoplognatha ovata (Clerck, 1757; Araneae: Theridiidae) 和E. latimana Hippa & Oksala, 1982 是不列颠群岛最常见的theridiid蜘蛛之一。在夏末,糖果条纹蜘蛛交配,雄性不久后死亡,而雌性则用丝卷活叶子(Eberhard et al., 2008),它们特有的蓝色卵囊沉积在其中,然后被保护直到第二龄前不久蜘蛛从卷叶中出现(牛津, 1993; 图1)。成熟雌性带卵囊的糖果条纹蜘蛛的数量被认为因气候、合适栖息地的可用性、干扰和其他因素而有很大差异,导致随着时间的推移,种群流量会增加 4 到 55 倍(Oxford, 1993 年)。在英国,从单个阔叶植被丛(牛津, 1993 年)中记录到多达 2744个E. ovata种群,估计多达 0.5 个成年或 2 个幼年Enoplognatha spp。在干旱灌木丛的麦田中,每平方米记录了蜘蛛(Opatovsky et al., 2016)。然而,根据栖息地的适宜性,区域和斑块之间的种群可能会有很大差异。叶卷,例如由糖果条纹蜘蛛为卵沉积而构建的叶卷,为未成熟的节肢动物带来了显着的好处,例如防止寄生和卵捕食(Kobayashi 等人, 2020 年;Loeffler, 1996 年;Tvardikova 和 Novotny, 2012 年)。然而,这些结构可能会通过意外消费(Tercel 等人, 2021 年)从视觉导向的捕食者(Danthanarayana, 1983 年;Kobayashi 等人, 2020 年)或无意的食草脊椎动物引入额外的捕食压力。

在这些叶卷中存在糖果条纹蜘蛛卵囊是基于合适的植物物种的存在。在农业背景下,这意味着它们对农作物害虫的潜在生物控制取决于农田附近的半自然栖息地的花卉群落组成。E. ovata长期以来一直以利用荆棘的叶子而闻名,但与蔷薇、榆树、枫树、榛、白蜡木、山毛榉、柳树、橡树、梅、荨麻、伞形花属、旋花属和各种其他植物的叶子的相互作用也已被发现记录(布里斯托, 1931 年;牛津, 1993 年)。这些偏好似乎超出了分类学,可能取决于叶子特征(Zanatta et al., 2022); 例如,Stevenson 和 Dindal ( 1982 ) 发现卷曲叶上的卵形 E. ovata幼虫多于平叶,尽管这是在落叶而不是活叶中。然而,将这种偏好扩展到植物物种,以及它如何影响卵子沉积的特征尚不清楚。尽管已知某些植物物种比其他物种使用得更多(例如,常见的荨麻Urtica dioica L., bramble Rubus fruticosus L.; Bristowe, 1931 ; Oxford, 1993),但在混合植物群落中,糖果条纹蜘蛛对这些植物的偏好,以及替代方案的适用性,人们知之甚少。
尽管像糖果条纹蜘蛛这样的theridiids经常出现在农作物中或农作物附近,但它们是非农业生物蜘蛛(即它们不会在作物本身中完成它们的生命周期),但仍然对许多农业生态系统的结网蜘蛛种群做出了重大贡献(Gavish-Regev 等人, 2008 年;Pluess 等人, 2008 年)。在英国, Enoplognatha ovata几乎没有作为生物防治剂进行过研究,但分子分析表明,这些蜘蛛经常早于英国作物中的叶蝉等害虫(Virant-Doberlet 等人, 2011 年)。在全球范围内,Enoplognatha属中的其他物种已被研究作为害虫甲虫的生物防治剂(Ranjbar Aghdam, 2013; Tóth 等人, 2002 年)鳞翅目昆虫(Luo 等人, 2014 年)、蚜虫(Gavish-Regev 等人, 2009 年)和飞虱(Chiu, 1979 年;Ganeshan, 2013 年)。Enoplognatha蜘蛛从邻近的栖息地迁移到农田,并表现出对蚜虫等害虫的高度偏好(Opatovsky 等人, 2012 年)。对于糖果条纹蜘蛛作为移民生物防治剂进入农作物,它们首先需要在邻近的半自然栖息地建立,这取决于提供合适的栖息地和寄主植物。
通过研究糖纹蜘蛛与其寄主植物和不同半自然栖息地之间的相互作用,可以阐明它们的偏好,这将对它们的生态学和生物防治潜力产生深远的影响。通过网络生态学的视角,可以进行此类分析,这是对复杂生态系统中生物群落之间相互作用的表征和评估(Bascompte, 2007)。经验网络生态学经常使用对野外物种相互作用的观察来构建相互作用网络,这是评估动物原位选择的有力手段(Cuff, Windsor, et al., 2022 ; Vaughan et al., 2018)。然而,动物和植物之间的相互作用主要是在对抗(例如,食草)或共生(例如,种子传播、授粉)背景下研究的,而不是在主要捕食性动物卷叶的共生背景下研究。
在这项研究中,我们将网络生态学原理和基于零模型的选择分析应用于对英格兰东北部糖果条纹蜘蛛叶和栖息地选择的观察,以提高我们对这些蜘蛛偏好的理解。我们检验了以下假设:(i)糖果条纹蜘蛛不成比例地利用树篱栖息地进行产卵,因为它们的灌木种类更加多样化;(ii) 糖果条纹蜘蛛在产卵时表现出对少数常见植物物种的偏好;(iii) 糖果条纹蜘蛛选择的用于产卵的植物具有相似的叶片形态。通过更好地了解这些蜘蛛的微生境偏好,可以调整农业管理和半自然栖息地可用性,以确保这些潜在重要的生物防治剂的增殖。










































 京公网安备 11010802027423号
京公网安备 11010802027423号