当前位置:
X-MOL 学术
›
J. Comput. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Endo- and exohedral complexes of superphane with cations
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2022-04-26 , DOI: 10.1002/jcc.26874 Mirosław Jabłoński 1
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2022-04-26 , DOI: 10.1002/jcc.26874 Mirosław Jabłoński 1
Affiliation
|
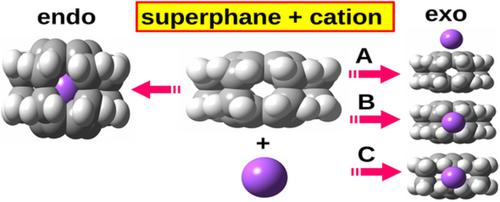
Quite recently it has been shown in a previous study that superphane, that is, [2.2.2.2.2.2](1,2,3,4,5,6)cyclophane, is a very convenient molecule in the study of endohedral complexes and especially in the study of the influence of the caged entity (i.e., guest) on the structure of the host molecule. This advantage results from the presence of two parallel benzene rings joined together by six quite flexible ethylene bridges (spacers). This article examines the energetic and structural properties of endo- and exohedral complexes of superphane with the following cations: H+, Li+, Na+, K+, Be2+, Mg2+, Ca2+, B3+, Al3+, Ga3+. The stability of endohedral complexes has been shown to be strongly dependent on the charge and radius of the caged cation. The inclusion of the cation inside the superphane molecule causes its ‘swelling’, which is manifested by an increase in the distance between the benzene rings and elongations of the ring and spacer C–C bonds. In the case of exohedral complexes, three forms are investigated: with the cation above the benzene ring, with the cation interacting with the superphane window in the equatorial position, and with the cation interacting with the center of the C–C spacer bond. The first of these forms has been shown to be preferred. The cation⋯acceptor distance depends on the cation radius. Among the cations investigated, H+ and Be2+ are particularly reactive and predisposed to induce significant structural changes in the superphane molecule, forming C–H bond or C–Be–C bridges, respectively.
中文翻译:

superphane 与阳离子的内面体和外面体配合物
最近的一项研究表明,superphane,即 [2.2.2.2.2.2](1,2,3,4,5,6)cyclophane,是研究内嵌配合物和特别是在研究笼状实体(即客体)对主体分子结构的影响方面。这种优势源于两个平行苯环的存在,它们通过六个相当灵活的乙烯桥(间隔物)连接在一起。本文研究了具有以下阳离子的超晶的内、外面体配合物的能量和结构特性:H +、Li +、Na +、K +、Be 2+、Mg 2+、Ca 2+、B 3+、Al 3+, Ga 3+。已显示内嵌络合物的稳定性强烈依赖于笼状阳离子的电荷和半径。将阳离子包含在超烷分子内会导致其“膨胀”,表现为苯环之间的距离增加以及环和间隔 C-C 键的伸长。在外面体配合物的情况下,研究了三种形式:阳离子在苯环上方,阳离子与赤道位置的超晶窗相互作用,阳离子与 C-C 间隔键的中心相互作用。这些形式中的第一种已被证明是优选的。阳离子⋯受体距离取决于阳离子半径。在所研究的阳离子中,H +和 Be 2+具有特别的反应性并易于引起超烷分子的显着结构变化,分别形成C-H键或C-Be-C桥。
更新日期:2022-04-26
中文翻译:

superphane 与阳离子的内面体和外面体配合物
最近的一项研究表明,superphane,即 [2.2.2.2.2.2](1,2,3,4,5,6)cyclophane,是研究内嵌配合物和特别是在研究笼状实体(即客体)对主体分子结构的影响方面。这种优势源于两个平行苯环的存在,它们通过六个相当灵活的乙烯桥(间隔物)连接在一起。本文研究了具有以下阳离子的超晶的内、外面体配合物的能量和结构特性:H +、Li +、Na +、K +、Be 2+、Mg 2+、Ca 2+、B 3+、Al 3+, Ga 3+。已显示内嵌络合物的稳定性强烈依赖于笼状阳离子的电荷和半径。将阳离子包含在超烷分子内会导致其“膨胀”,表现为苯环之间的距离增加以及环和间隔 C-C 键的伸长。在外面体配合物的情况下,研究了三种形式:阳离子在苯环上方,阳离子与赤道位置的超晶窗相互作用,阳离子与 C-C 间隔键的中心相互作用。这些形式中的第一种已被证明是优选的。阳离子⋯受体距离取决于阳离子半径。在所研究的阳离子中,H +和 Be 2+具有特别的反应性并易于引起超烷分子的显着结构变化,分别形成C-H键或C-Be-C桥。











































 京公网安备 11010802027423号
京公网安备 11010802027423号