当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A sobering examination of the feasibility of aqueous aluminum batteries
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2022-04-23 , DOI: 10.1039/d2ee00134a Glenn R. Pastel 1 , Ying Chen 2 , Travis P. Pollard 1 , Marshall A. Schroeder 1 , Mark E. Bowden 2 , Allen Zheng 3 , Nathan T. Hahn 4 , Lin Ma 1 , Vijayakumar Murugesan 2 , Janet Ho 1 , Mounesha Garaga 3 , Oleg Borodin 1 , Karl Mueller 2 , Steven Greenbaum 3 , Kang Xu 1
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2022-04-23 , DOI: 10.1039/d2ee00134a Glenn R. Pastel 1 , Ying Chen 2 , Travis P. Pollard 1 , Marshall A. Schroeder 1 , Mark E. Bowden 2 , Allen Zheng 3 , Nathan T. Hahn 4 , Lin Ma 1 , Vijayakumar Murugesan 2 , Janet Ho 1 , Mounesha Garaga 3 , Oleg Borodin 1 , Karl Mueller 2 , Steven Greenbaum 3 , Kang Xu 1
Affiliation
|
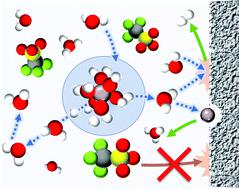
Aqueous aluminum (Al) batteries are posited to be a cheap and energy dense alternative to conventional Li-ion chemistries, but an aqueous electrolyte mediating trivalent aluminum cations (Al3+) warrants greater scrutiny. This study provides a rigorous examination of aqueous Al electrolytes, with the first compelling evidence for a dynamic octahedral solvation structure around the Al3+, without Al–OTf contact ion pairs, even at high concentrations. This solvation behavior and the concomitant, transient electrostatic hydrolysis of Al–OH2 ligands contrasts strongly with previously reported water-in-salt electrolytes, and occurs due to the high charge density of the Lewis acidic Al3+. Nuclear magnetic resonance spectroscopy and other physicochemical measurements quantitatively reveal how species activity evolves with concentration and temperature. This new understanding exposes practical concerns related to the corrosiveness of the acidic aqueous solutions, the degree of hydration of aluminum trifluoromethanesulfonate (Al(OTf)3) salt, and the grossly insufficient reductive stability of the proposed electrolytes (>1 V between HER onset and Al3+/Al). Collectively, these factors constitute multiple fundamental barriers to the feasibility of rechargeable aqueous Al batteries.
中文翻译:

水性铝电池可行性的清醒检验
水性铝 (Al) 电池被认为是传统锂离子化学物质的廉价且能量密集的替代品,但介导三价铝阳离子 (Al 3+ ) 的水性电解质需要更严格的审查。这项研究对水性铝电解质进行了严格的检验,第一个令人信服的证据表明,即使在高浓度下, Al 3+周围也存在动态八面体溶剂化结构,没有 Al-OTf 接触离子对。这种溶剂化行为和伴随的 Al-OH 2配体的瞬时静电水解与先前报道的盐包水电解质形成强烈对比,并且由于路易斯酸性 Al 3+的高电荷密度而发生. 核磁共振光谱和其他物理化学测量定量地揭示了物种活动如何随着浓度和温度而演变。这种新的认识揭示了与酸性水溶液的腐蚀性、三氟甲磺酸铝 (Al(OTf) 3 ) 盐的水合程度以及所提出的电解质的还原稳定性严重不足(在 HER 开始和 HER 开始之间>1 V)相关的实际问题Al 3+ /Al)。总的来说,这些因素构成了可充电水性铝电池可行性的多个基本障碍。
更新日期:2022-04-23
中文翻译:

水性铝电池可行性的清醒检验
水性铝 (Al) 电池被认为是传统锂离子化学物质的廉价且能量密集的替代品,但介导三价铝阳离子 (Al 3+ ) 的水性电解质需要更严格的审查。这项研究对水性铝电解质进行了严格的检验,第一个令人信服的证据表明,即使在高浓度下, Al 3+周围也存在动态八面体溶剂化结构,没有 Al-OTf 接触离子对。这种溶剂化行为和伴随的 Al-OH 2配体的瞬时静电水解与先前报道的盐包水电解质形成强烈对比,并且由于路易斯酸性 Al 3+的高电荷密度而发生. 核磁共振光谱和其他物理化学测量定量地揭示了物种活动如何随着浓度和温度而演变。这种新的认识揭示了与酸性水溶液的腐蚀性、三氟甲磺酸铝 (Al(OTf) 3 ) 盐的水合程度以及所提出的电解质的还原稳定性严重不足(在 HER 开始和 HER 开始之间>1 V)相关的实际问题Al 3+ /Al)。总的来说,这些因素构成了可充电水性铝电池可行性的多个基本障碍。











































 京公网安备 11010802027423号
京公网安备 11010802027423号