当前位置:
X-MOL 学术
›
Asia Pac. J. Clin. Oncol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Clinical efficacy and safety of pemetrexed with or without either Bevacizumab or Pembrolizumab in patients with metastatic nonsquamous non–small cell carcinoma
Asia-Pacific Journal of Clinical Oncology ( IF 1.4 ) Pub Date : 2022-04-20 , DOI: 10.1111/ajco.13658 Wang Chun Kwok 1 , Tan Fong Cheong 2 , Ka Yan Chiang 1 , James Chung Man Ho 1 , David Chi Leung Lam 1 , Mary Sau Man Ip 1 , Terence Chi Chun Tam 1
Asia-Pacific Journal of Clinical Oncology ( IF 1.4 ) Pub Date : 2022-04-20 , DOI: 10.1111/ajco.13658 Wang Chun Kwok 1 , Tan Fong Cheong 2 , Ka Yan Chiang 1 , James Chung Man Ho 1 , David Chi Leung Lam 1 , Mary Sau Man Ip 1 , Terence Chi Chun Tam 1
Affiliation
|
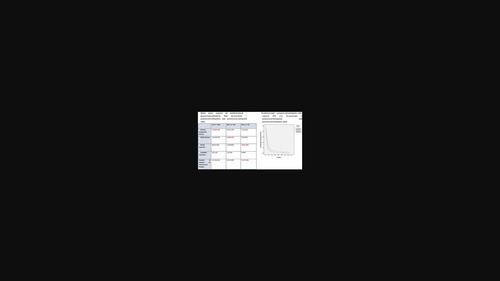
Pemetrexed was approved by United States Food and Drug Administration (US FDA) in combination with platinum for the treatment of advanced nonsquamous non–small cell lung carcinoma (NSCLC) and malignant mesothelioma. Bevacizumab and pembrolizumab can be added to chemotherapy for patients with nonsquamous NSCLC with benefits but there has not been any dedicated head-to-head comparison between pembrolizumab-pemetrexed-platinum (PAC) and bevacizumab-pemetrexed-platinum (BAC) on their efficacy and safety.
中文翻译:

培美曲塞联合或不联合贝伐单抗或派姆单抗治疗转移性非鳞状非小细胞癌的临床疗效和安全性
培美曲塞被美国食品和药物管理局 (US FDA) 批准与铂类联合用于治疗晚期非鳞状非小细胞肺癌 (NSCLC) 和恶性间皮瘤。贝伐珠单抗和派姆单抗可加入非鳞状 NSCLC 患者的化疗中获益,但派姆单抗-培美曲塞-铂 (PAC) 和贝伐珠单抗-培美曲塞-铂 (BAC) 的疗效和疗效之间尚未进行任何专门的头对头比较安全。
更新日期:2022-04-20
中文翻译:

培美曲塞联合或不联合贝伐单抗或派姆单抗治疗转移性非鳞状非小细胞癌的临床疗效和安全性
培美曲塞被美国食品和药物管理局 (US FDA) 批准与铂类联合用于治疗晚期非鳞状非小细胞肺癌 (NSCLC) 和恶性间皮瘤。贝伐珠单抗和派姆单抗可加入非鳞状 NSCLC 患者的化疗中获益,但派姆单抗-培美曲塞-铂 (PAC) 和贝伐珠单抗-培美曲塞-铂 (BAC) 的疗效和疗效之间尚未进行任何专门的头对头比较安全。











































 京公网安备 11010802027423号
京公网安备 11010802027423号