当前位置:
X-MOL 学术
›
Chem. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Anion-Exchange Membrane Water Electrolyzers
Chemical Reviews ( IF 51.4 ) Pub Date : 2022-04-20 , DOI: 10.1021/acs.chemrev.1c00854 Naiying Du 1, 2 , Claudie Roy 2, 3 , Retha Peach 4 , Matthew Turnbull 1, 2 , Simon Thiele 4, 5 , Christina Bock 1, 2
Chemical Reviews ( IF 51.4 ) Pub Date : 2022-04-20 , DOI: 10.1021/acs.chemrev.1c00854 Naiying Du 1, 2 , Claudie Roy 2, 3 , Retha Peach 4 , Matthew Turnbull 1, 2 , Simon Thiele 4, 5 , Christina Bock 1, 2
Affiliation
|
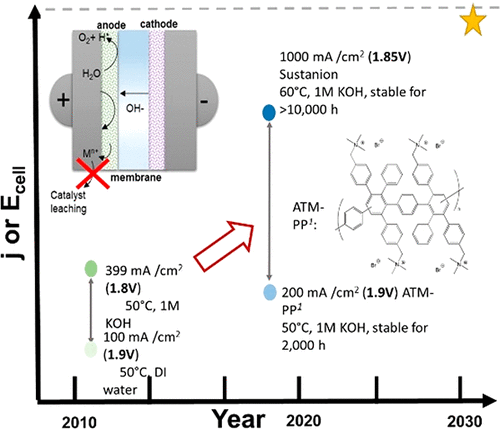
This Review provides an overview of the emerging concepts of catalysts, membranes, and membrane electrode assemblies (MEAs) for water electrolyzers with anion-exchange membranes (AEMs), also known as zero-gap alkaline water electrolyzers. Much of the recent progress is due to improvements in materials chemistry, MEA designs, and optimized operation conditions. Research on anion-exchange polymers (AEPs) has focused on the cationic head/backbone/side-chain structures and key properties such as ionic conductivity and alkaline stability. Several approaches, such as cross-linking, microphase, and organic/inorganic composites, have been proposed to improve the anion-exchange performance and the chemical and mechanical stability of AEMs. Numerous AEMs now exceed values of 0.1 S/cm (at 60–80 °C), although the stability specifically at temperatures exceeding 60 °C needs further enhancement. The oxygen evolution reaction (OER) is still a limiting factor. An analysis of thin-layer OER data suggests that NiFe-type catalysts have the highest activity. There is debate on the active-site mechanism of the NiFe catalysts, and their long-term stability needs to be understood. Addition of Co to NiFe increases the conductivity of these catalysts. The same analysis for the hydrogen evolution reaction (HER) shows carbon-supported Pt to be dominating, although PtNi alloys and clusters of Ni(OH)2 on Pt show competitive activities. Recent advances in forming and embedding well-dispersed Ru nanoparticles on functionalized high-surface-area carbon supports show promising HER activities. However, the stability of these catalysts under actual AEMWE operating conditions needs to be proven. The field is advancing rapidly but could benefit through the adaptation of new in situ techniques, standardized evaluation protocols for AEMWE conditions, and innovative catalyst-structure designs. Nevertheless, single AEM water electrolyzer cells have been operated for several thousand hours at temperatures and current densities as high as 60 °C and 1 A/cm2, respectively.
中文翻译:

阴离子交换膜水电解槽
本综述概述了用于带有阴离子交换膜 (AEM) 的水电解槽(也称为零间隙碱性水电解槽)的催化剂、膜和膜电极组件 (MEA) 的新兴概念。最近的进展很大程度上归功于材料化学、MEA 设计和优化操作条件的改进。阴离子交换聚合物(AEP)的研究主要集中在阳离子头/主链/侧链结构以及离子电导率和碱性稳定性等关键性能上。人们提出了几种方法,例如交联、微相和有机/无机复合材料来提高AEM的阴离子交换性能以及化学和机械稳定性。目前,许多 AEM 的值都超过了 0.1 S/cm(在 60–80 °C 时),但在超过 60 °C 的温度下的稳定性还需要进一步增强。析氧反应(OER)仍然是一个限制因素。对薄层 OER 数据的分析表明 NiFe 型催化剂具有最高的活性。关于 NiFe 催化剂的活性位点机制存在争议,需要了解其长期稳定性。在 NiFe 中添加 Co 可以提高这些催化剂的电导率。对析氢反应 (HER) 的相同分析显示碳负载的 Pt 占主导地位,尽管 PtNi 合金和 Pt 上的 Ni(OH) 2簇表现出竞争活性。在功能化高表面积碳载体上形成和嵌入分散良好的 Ru 纳米颗粒的最新进展显示出有前景的 HER 活性。然而,这些催化剂在实际 AEMWE 操作条件下的稳定性需要得到证明。 该领域正在迅速发展,但可以通过采用新的原位技术、AEMWE 条件的标准化评估协议和创新的催化剂结构设计而受益。尽管如此,单个 AEM 水电解池已分别在高达 60 °C 和 1 A/cm 2的温度和电流密度下运行了数千小时。
更新日期:2022-04-20
中文翻译:

阴离子交换膜水电解槽
本综述概述了用于带有阴离子交换膜 (AEM) 的水电解槽(也称为零间隙碱性水电解槽)的催化剂、膜和膜电极组件 (MEA) 的新兴概念。最近的进展很大程度上归功于材料化学、MEA 设计和优化操作条件的改进。阴离子交换聚合物(AEP)的研究主要集中在阳离子头/主链/侧链结构以及离子电导率和碱性稳定性等关键性能上。人们提出了几种方法,例如交联、微相和有机/无机复合材料来提高AEM的阴离子交换性能以及化学和机械稳定性。目前,许多 AEM 的值都超过了 0.1 S/cm(在 60–80 °C 时),但在超过 60 °C 的温度下的稳定性还需要进一步增强。析氧反应(OER)仍然是一个限制因素。对薄层 OER 数据的分析表明 NiFe 型催化剂具有最高的活性。关于 NiFe 催化剂的活性位点机制存在争议,需要了解其长期稳定性。在 NiFe 中添加 Co 可以提高这些催化剂的电导率。对析氢反应 (HER) 的相同分析显示碳负载的 Pt 占主导地位,尽管 PtNi 合金和 Pt 上的 Ni(OH) 2簇表现出竞争活性。在功能化高表面积碳载体上形成和嵌入分散良好的 Ru 纳米颗粒的最新进展显示出有前景的 HER 活性。然而,这些催化剂在实际 AEMWE 操作条件下的稳定性需要得到证明。 该领域正在迅速发展,但可以通过采用新的原位技术、AEMWE 条件的标准化评估协议和创新的催化剂结构设计而受益。尽管如此,单个 AEM 水电解池已分别在高达 60 °C 和 1 A/cm 2的温度和电流密度下运行了数千小时。











































 京公网安备 11010802027423号
京公网安备 11010802027423号