Hydrometallurgy ( IF 4.8 ) Pub Date : 2022-04-20 , DOI: 10.1016/j.hydromet.2022.105886 Florian Verbruggen 1, 2 , Antonin Prévoteau 1, 2 , Luiza Bonin 1, 2 , Kristof Marcoen 3 , Tom Hauffman 3 , Tom Hennebel 1, 2, 4 , Korneel Rabaey 1, 2 , Michael S. Moats 5
|
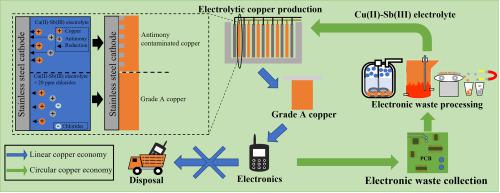
The use of electronic waste or low grade materials as feedstock for the electrolytic production of copper is challenging because impurity metals such as Sb(III) are introduced in the electrolyte. In this work, the mechanisms that lead to antimony contamination in electrolytic copper are studied. Linear sweep voltammetry experiments indicate that the reduction of Sb(III) is kinetically slow in the absence of Cu(II). In the presence of Cu(II), however, reduction of Sb(III) can occur readily by the codeposition of Cu(II) and Sb(III) as demonstrated by chronoamperometry. The ToF-SIMS analyses confirmed the codeposition of antimony in the very first micrometer of the copper deposit, enabled by the nucleation overpotential for galvanostatic copper electrodeposition under conditions relevant for the commercial production of copper. Based on potentiostatic electrodeposition experiments, we suggest that a copper concentration of ≥40 g L−1 Cu(II) in Sb(III) containing electrolytes is beneficial to obtain high purity copper. Codeposition reactions were impacted by the presence of additives (thiourea, glue and chloride ions). In particular, the addition of 0.02 g L−1 chloride mitigated the codeposition of antimony (0.02 g L−1 Sb(III)) to produce grade A copper. For optimal removal of Sb(III) from bleed electrolytes, a molar ratio of ~3 Cu(II)/Sb(III) should be maintained (e.g. 0.3 g L−1 Cu(II) for a typical concentration of 0.2 g L−1 Sb(III)).
中文翻译:

铜锑的电化学共沉积及其与电解质添加剂的相互作用:将电子废物用于可持续的铜电冶金
使用电子废料或低品位材料作为电解生产铜的原料具有挑战性,因为电解液中会引入 Sb(III) 等杂质金属。在这项工作中,研究了导致电解铜中锑污染的机制。线性扫描伏安法实验表明,在没有 Cu(II) 的情况下,Sb(III) 的还原动力学缓慢。然而,在存在 Cu(II) 的情况下,Sb(III) 的还原很容易通过 Cu(II) 和 Sb(III) 的共沉积发生,如计时电流法所示。ToF-SIMS 分析证实了锑在铜矿床的第一微米中的共沉积,这是由于在与铜的商业生产相关的条件下,恒电流铜电沉积的成核过电位所致。-1 Cu(II) 在含 Sb(III) 的电解液中有利于获得高纯度铜。共沉积反应受到添加剂(硫脲、胶和氯离子)的影响。特别是,添加 0.02 g L -1氯化物减轻了锑 (0.02 g L -1 Sb(III)) 的共沉积以生产 A 级铜。为了从渗出电解质中最佳去除 Sb(III),应保持约 3 Cu(II)/Sb(III) 的摩尔比(例如,0.3 g L -1 Cu(II),典型浓度为 0.2 g L - 1 Sb(III))。











































 京公网安备 11010802027423号
京公网安备 11010802027423号